09 May 2024: Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate Coronary Artery Lesions
Piotr BaruśDOI: 10.12659/MSM.943956
Med Sci Monit 2024; 30:e943956
Abstract
BACKGROUND: Progression of chronic coronary syndrome (CCS) is influenced by chronic kidney disease (CKD). This 5-year follow-up study aimed to assess 100 patients with 118 intermediate coronary artery lesions evaluated by fractional flow reserve (FFR) and intravascular imaging stratified according to renal function.
MATERIAL AND METHODS: This prospective study enrolled patients with intermediate coronary stenosis identified by coronary angiogram. Patients with severe renal dysfunction (estimated glomerular filtration rate (eGFR) <45 ml/min/1.73 m²) were excluded from the study. The remaining were divided into 2 groups according to eGFR: 45-60 ml/min/1.73 m² for mild-to-moderate renal dysfunction and >60 ml/min/1.73 m² for no renal dysfunction. We analyzed intermediate-grade stenoses (40-80% as assessed in coronary angiography) with the use of optical coherence tomography (OCT), FFR, and intravascular ultrasound (IVUS).
RESULTS: Renal dysfunction patients were older (67.7±8.1 vs 63.6±9.7 years, P=0.044). Lesion characteristics, including plaque type and minimal lumen area in OCT, showed no significant differences between the renal dysfunction and no renal dysfunction groups. Thin-cap fibroatheroma, calcific plaques, lipidic plaques, and fibrous plaques had similar prevalence. FFR values and IVUS parameters did not significantly differ between the groups. Over a 5-year follow-up, individuals with mild-to-moderate renal dysfunction had an elevated risk of all-cause mortality and major adverse cardiovascular events in multivariate analyses adjusted for age and sex.
CONCLUSIONS: Mild-to-moderate renal dysfunction was not associated with significant differences in OCT- and IVUS-derived plaque morphology nor with functional indices characterizing intermediate-grade coronary stenoses. Renal dysfunction was related to a higher risk of all-cause mortality and major adverse cardiovascular events prevalence in 5-year follow-up.
Keywords: Ultrasonography, Interventional, Tomography, Optical Coherence, Coronary Artery Disease, Fractional Flow Reserve, Myocardial
Introduction
Progression of chronic coronary syndrome (CCS), namely ongoing atherosclerotic plaque formation and risk of plaque destabilization, depends on various factors, including comorbidities [1–3]. Chronic kidney disease (CKD) is associated with a worse prognosis in patients with CCS [1,4,5]. Several studies have shown the association between deteriorating renal function and an increased risk of acute coronary syndrome (ACS), as well as a higher prevalence of major adverse cardiovascular events (MACE) after percutaneous coronary intervention (PCI) [1,4,5]. Moreover, atherosclerosis has been proven to develop more rapidly in patients with CKD, especially those with end-stage kidney disease [6,7]. Optical coherence tomography (OCT), fractional flow reserve (FFR), and intravascular ultrasound (IVUS) of intermediate-grade coronary stenoses (40–80%) [8,9] can provide meticulous information regarding plaque composition and influence on hemodynamic function [10–13]. In severe CKD patients, a larger amount of calcium is observed and the calcified plaque is more prevalent [14–19], demanding a different approach during PCI, including the use of high-pressure balloons, cutting balloons, intravascular lithotripsy, or rotablation [20]. CKD can also lead to higher FFR values [21]. There is limited information on specific, multimodality-derived plaque characteristics in patients with mild-to-moderate renal dysfunction and the association with follow-up.
Due to the widely recognized limitations of coronary angiography in determining hemodynamic significance of ambiguous coronary stenoses, functional lesion assessment is recommended [22–24] along with intravascular imaging, and this had gained increasing interest in the management of coronary syndromes [25–32]. FFR, a wire-based technique, is performed during cardiac catheterization, measuring the pressure before and after the stenosis in an isolated segment of the vessel during maximal hyperemia [33], which is usually induced by intravenous administration of adenosine [34]. The result is presented as a ratio of pressure distal to the stenosis divided by pressure proximal to the stenosis and is a useful tool for identifying intermediate-grade lesions significantly restricting blood flow [35]. OCT has some distinctive features, including high resolution (axial 10–20 μm and lateral 20–90 μm) and penetration depth ranging from 1 to 2 mm [25]. It thus allows a thorough analysis of the superficial vessel layers, and a detailed coronary plaque assessment is compromised by imperfect visualization of the deeper layers of the coronary artery [25,36–42]. Intravascular ultrasound (IVUS) has a tissue penetration depth of 5–6 mm, allowing visualization of the full vessel wall thickness, including the external elastic membrane (EEM), the diameter of which serves as a guide for stenting optimization, but with a lower resolution (axial 100–150 μm) than OCT [26,36,37,42–44]. Additionally, the ability to visualize all layers of the arterial wall enables assessment of vessel remodeling and plaque burden [37,45].
Research is ongoing regarding high-risk plaque characteristics, which in the long term are likely to cause MACE [28,46–53]. Assessing coronary arteries multimodally allows for acquisition of data regarding plaque morphology and functional indices [28,47,50–53]. Patients with CKD are more susceptible to faster progression of CCS [1,4,5]. There is limited information on the impact of mild-to-moderate renal dysfunction on intermediate-grade stenoses in coronary arteries in relation to long-term follow-up. The aim of this 5-year follow-up study was to assess the morphometric (OCT, IVUS) and functional (FFR) parameters of intermediate coronary artery lesions evaluated during cardiac catheterization in patients with renal dysfunction vs those without renal dysfunction.
Material and Methods
ETHICS APPROVAL AND INFORMED CONSENT:
Approval for this prospective study was granted by the Ethics Committee at the Medical University of Warsaw (approval no. 1432020), and all procedures were conducted in adherence to the principles outlined in the Declaration of Helsinki. Prior to participation, patients provided informed and written consent.
STUDY POPULATION:
This was a single-center, prospective, observational, longitudinal cohort study, which consecutively enrolled 100 patients (118 lesions) with CCS undergoing a coronary angiogram (CAG) in a tertiary heart center (ClinicalTrials.gov registration nr: NCT06261866) [54,55]. Intermediate-grade coronary lesions were analyzed by a multimodality approach using OCT and FFR, and with IVUS in a subset of patients. Stenoses were classified as significant if FFR was ≤0.8. Patients were divided into 2 groups according to the estimated glomerular filtration rate (eGFR) level: 45–60 ml/min/1.73 m2 for mild-to-moderate renal dysfunction and >60 ml/min/1.73 m2 for no renal dysfunction.
The inclusion criteria were: CCS defined as a positive result of an ischemic test (exercise test or single photon emission tomography (SPECT)) or prevalence and severity of chest pain symptoms ranked according to the Canadian Cardiovascular Society classification (CCS 2–3), age >18 years, and intermediate coronary stenoses defined as a stenosis of 40–80% evaluated visually in CAG [9]. Exclusion criteria were: hemodynamic instability, bypass graft or ostial left main or ostial right coronary lesions, severely impaired renal function defined as eGFR <45 ml/min/1.73 m2, contraindication for adenosine administration, and pregnancy.
OPTICAL COHERENCE TOMOGRAPHY:
OCT recordings and data acquisition were performed utilizing an OCT imaging system (Abbott, C7XR Dragonfly TM, LightLab Imaging, Inc., MA, USA). Images were obtained using the non-occlusive technique [22,25,41,42,56,57]. This technique does not require the use of a balloon to block the coronary blood flow for the image acquisition; instead, a quick flush of contrast is administered into the coronary artery, thus blood morphological elements do not cause artifacts [56,57].
Current expert consensuses [58–61] served as guidelines for OCT images analysis. Analysts were kept unaware of FFR, IVUS results, and patients’ characteristics. Evaluation of the reference lumen area involved assessment of the largest lumen either proximal or distal to a stenosis (within 10 mm of the stenosis). Morphometric assessment of plaques was conducted at the site of minimal lumen area (MLA) over at least 3 consecutive frames. Plaques were categorized as calcified, fibrous, lipid-rich, or mixed. Calcified plaques exhibit a signal-poor heterogeneous region with sharply delineated border [58–61]. We also assessed the calcium angle within the plaque. A fibrous plaque is defined by high backscattering and a relatively homogenous signal [58–61]. A plaque was deemed lipid-rich when it presented an irregular signal-poor region with diffused borders (Figure 1) [59,62]. The lipid angle was calculated as the degree measure of a low-signal region. Fibrous cap thickness (FCT) refers to the distance between the inner edge of the lipid or calcium pool and vessel lumen border. FCT measurements were taken at 0.2-mm intervals across the plaque. Subsequently, 3 measurements were taken at the thinnest part of the plaque at each cross-section, and the average value was considered for the final analyses [62]. A plaque with minimal FCT <65 μm was classified as a thin-cap fibroatheroma (TCFA) [61].
FRACTIONAL FLOW RESERVE:
The coronary pressure analysis was performed with a commercially available 0.014-inch pressure guide wire (St. Jude Medical, Minneapolis, MN, USA). The pressure wire was placed into the coronary artery, then the tip of the pressure wire was accurately positioned 1–2 mm outside of the catheter to equalize pressure [63]. Afterwards, the pressure wire was placed distally to the assessed lesion [63] and maximal hyperemia was induced by administering adenosine intravenously at a rate of 140 μg/kg/min through a large peripheral vein [34]. FFR was determined by dividing the mean hyperemic distal coronary pressure by the mean aortic pressure. A lesion was considered significant if the FFR was ≤0.8 [12,13,64–66].
INTRAVASCULAR ULTRASOUND:
For IVUS image acquisition, a catheter was positioned in the distal section of the coronary vessel and a pullback was conducted at a speed of 0.5 mm/s at 40 MHz [67]. Image evaluation occurred at 0.5-mm intervals utilizing specialized software, with the analyst being blinded to patients’ FFR and OCT results, as well as clinical characteristics. The plaque burden at the site of MLA was computed using the formula: [68].
FOLLOW-UP:
The primary endpoint was MACE defined as a composite of cardiac death, myocardial infarction, stroke, unplanned PCI, coronary artery bypass grafting and hospitalization due to heart failure. Cardiac death was defined as death due to CCS, ACS, or cardiac arrest. The secondary endpoint was all-cause death. The follow-up was obtained by on-site visits, through data obtained in national and hospital registries, and by phone if necessary.
STATISTICAL ANALYSIS:
Statistical analyses were conducted using SPSS version 28.0 (IBM Corp, Armonk, NY, USA). The distribution of collected data was assessed through the Kolmogorov-Smirnov test. For normally distributed data, means and standard deviations (SD) were presented, while non-parametric data were represented by median values and the interquartile range (IQR) between the 25th and 75th percentiles. Categorical data were expressed as percentages within respective groups. Group comparisons involved the
Results
PATIENTS’ CHARACTERISTICS:
This study enrolled 100 patients (118 lesions) with a mean age of 64.5±9.5 years. Patients were divided into 2 groups – renal dysfunction (n=23) and no renal dysfunction (n=77) – according to the eGFR level: 45–60 ml/min/1.73 m2 for mild-to-moderate renal dysfunction and >60 ml/min/1.73 m2 for no renal dysfunction. The median value of eGFR in both groups was 54.9±3.9 ml/min/1.73 m2 vs 85.8±17.1 ml/min/1.73 m2, for renal dysfunction and no renal dysfunction, respectively (
The baseline characteristics are presented in Table 1. Patients with renal dysfunction were older (67.7±8.1 vs 63.6±9.7 years, P=0.044). No significant dissimilarities were found regarding comorbidities or laboratory test results (except creatinine level 1.25±0.16 mg/dl vs 0.89±0.16 mg/dl, renal dysfunction vs no renal dysfunction respectively, P<0.001) between groups. Patients without renal dysfunction were more frequently treated with β-blockers (97.4% vs 87.0%, P=0.044) (Table 1).
FRACTIONAL FLOW RESERVE:
All lesions were assessed by FFR. The plaque location did not manifest significant dissimilarities, and in both groups most frequently appeared in the left anterior descending artery. There was a trend in difference in FRR values between renal dysfunction patients and no renal dysfunction patients (0.81±0.11 vs 0.77±0.12, respectively,
OPTICAL COHERENCE TOMOGRAPHY:
In OCT analysis, no differences were shown regarding plaque type in both groups. Among renal dysfunction patients, the 2 most prevalent plaque types were fibrous and calcified (both: n=11, 36.7%). On the contrary, the most common plaque type in patients without renal dysfunction was calcified (n=32, 37.2%) (Table 2).
There were no dissimilarities regarding lesion length, minimal lumen diameter, minimal lumen area, mean lumen area, cap thickness over calcium, angle of calcium, presence of TCFA, and thrombus (Table 3).
INTRAVASCULAR ULTRASOUND:
In a subset of 57 lesions, IVUS was performed. As with OCT, there was no statistically significant difference in lesion length, lumen volume, plaque volume, lumen area, vessel area, or plaque burden between groups (Table 4).
FOLLOW-UP:
Clinical follow-up data on mortality were available for 95 study participants. The median follow-up time was 55.5±12.5 months. Presence of mild-to-moderate renal dysfunction in 5-year long follow-up was linked to an increased risk of mortality from any cause (33.3% vs 12.2%, HR=3.16, 95%CI [1.18, 8.51], P =0.016) in multivariate analyses adjusted for age and sex (Figure 2).
Follow-up regarding MACE (defined as cardiac death, myocardial infarction, stroke, unplanned PCI, coronary artery bypass grafting, or hospitalization due to heart failure) was available for 62 patients (Table 5). The median follow-up time was 45.8±20.3 months. MACE in 5-year follow-up occurred in 70.6% of patients with mild-to-moderate renal dysfunction compared to 36.6% of patients without renal dysfunction (HR=2.54, 95% CI: 1.20–5.38, P=0.011 in a multivariate analysis adjusted for age and sex) (Figure 3).
Discussion
The main findings of this study can be summarized as follows: i) Patients suffering from CCS with mild-to-moderate renal dysfunction did not present significant differences in OCT-detected plaque morphology and IVUS-obtained parameters compared to individuals without renal dysfunction; ii) There was a trend suggesting that renal dysfunction patients tend to have overall higher FFR values; iii) Renal dysfunction was linked to elevated risk of all-cause mortality and increased risk of MACE in 5-year follow-up.
A study by Tebaldi et al that enrolled 1004 patients, including 131 with eGFR values of ≤45 ml/min/1.73 m2, showed that patients with CKD present significantly higher FFR values in comparison to people with mild-to-moderate renal dysfunction or no renal dysfunction (0.84±0.07 vs 0.81±0.08,
In our IVUS analysis, no differences were established regarding: lumen volume, plaque volume, lumen area, plaque area, plaque burden, and calcification angle. A study by Miyagi et al divided 89 patients with stable angina into 2 groups in relation to eGFR with a cut-off point at 60 ml/min/1.73 m2, and they had a negative correlation with lipid volume [18]. Two studies have concentrated on IVUS evaluation of coronary plaques in hemodialyzed patients and showed more calcifications and a greater plaque volume in this group [17,19]. The primary difference between those studies in relation to our study is the enrollment criteria. Hemodialyzed patients are an extreme example of chronic kidney disease with minimal eGFR values; in contrast, the mean eGFR value in the renal dysfunction group in our study was 54.9±3.9 ml/min/1.73 m2.
We found no statistically significant differences regarding OCT-defined plaque types in renal dysfunction vs no renal dysfunction patients. Several studies have found more frequent calcifications among patients with CKD [14–16]. Of note, however, those studies concentrated on patients with end-stage renal disease and hemodialyzed patients, whereas our study concentrated on people with mild-to-moderate renal dysfunction. As such associations were not found in our study, it is crucial to further deepen the research regarding the number of calcifications among people with CKD, stratifying the participants into subgroups based on eGFR levels. A study be Sugiyama et al was designed to compare OCT-derived plaque parameters in different CKD stages. In patients presenting with CCS, 296 native coronary lesions treated with stent implantation were assessed. Lesions were divided into 3 groups: a non-CKD group (eGFR≥60 ml/min/1.73 m2), a CKD group (eGFR 15–60 ml/min/1.73 m2), and an end-stage kidney disease (ESKD) group (eGFR<15 ml/min/1.73 m2). Lipid-rich plaques were more frequent in the CKD group (
In summary, most studies to date describing the relationship between impaired renal function and atherosclerotic plaque characteristics have focused on patients with advanced-stage CKD. They have demonstrated more frequent calcifications among CKD patients, which may require a different strategy (eg, high-pressure balloons, cutting balloons, intravascular lithotripsy, or rotablation) in case of PCI [20]. However, in our study such results were not presented; therefore, when performing a PCI in patients with mild-to-moderate renal dysfunction, one can expect a comparable number of calcifications to patients with normal renal function.
In the follow-up analysis, mild-to-moderate renal dysfunction was associated with increased all-cause mortality and a higher incidence of MACE during the 5-year observation. CKD is a well-established comorbidity that worsens prognosis [1,4–7,24,40,54,74].
The primary limitation of our study is a relatively small patient sample size and conducting it at only 1 site. Moreover, the study was restricted to only CCS and this data cannot be directly compared to patients with ACS. Several anatomical features (eg, bifurcations, ostial lesions, tortuous vessels) might have influenced the accuracy of OCT, FFR, and IVUS measurements. Moreover, when evaluating OCT or IVUS images, there might be heterogeneity between analysts, as the parameters are not computed by a program but the borders of different structures are marked by a human. Because the FFR result was decisive if PCI was performed, one can suspect that some patients may have received suboptimal treatment and high-risk plaques might have not been stented. Also, limited data were available in terms of MACE follow-up, and further studies on larger cohorts are warranted.
Conclusions
Mild-to-moderate renal dysfunction was not associated with significant differences in OCT- and IVUS-derived plaque morphology nor with functional indices characterizing intermediate-grade coronary stenoses among patients with CCS. Mild-to-moderate renal dysfunction was associated with higher risk of all-cause mortality and higher MACE prevalence in 5-year follow-up.
Figures
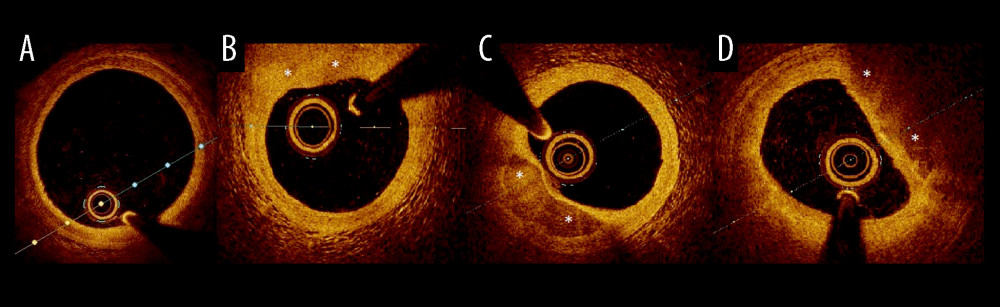 Figure 1. Examples of plaque types in optical coherence tomography. (A) Healthy vessel (normal three-layer artery wall visible), (B) Fibrous plaques (homogenous signal, high backscattering), (C) Calcified plaque (signal-poor heterogeneous region with sharply delineated border), (D) lipid-rich plaque (inhomogeneous signal-poor region with diffused borders). Plaques are marked with an *.
Figure 1. Examples of plaque types in optical coherence tomography. (A) Healthy vessel (normal three-layer artery wall visible), (B) Fibrous plaques (homogenous signal, high backscattering), (C) Calcified plaque (signal-poor heterogeneous region with sharply delineated border), (D) lipid-rich plaque (inhomogeneous signal-poor region with diffused borders). Plaques are marked with an *. ![Kaplan-Meier curve for overall survival (months) comparing patients (n=95) with renal dysfunction (estimated glomerular filtration rate 45–60 ml/min/1.73 m2) and without renal dysfunction (estimated glomerular filtration rate >60 ml/min/1.73 m2). Presence of mild-to-moderate renal dysfunction, in 5-year follow-up was associated with higher all-cause mortality (33.3% vs 12.2%, HR=3.16, 95%CI [1.18, 8.51], P=0.016) in multivariate analyses adjusted for age and sex. Log-rank P=0.016.](https://jours.isi-science.com/imageXml.php?i=medscimonit-30-e943956-g002.jpg&idArt=943956&w=1000) Figure 2. Kaplan-Meier curve for overall survival (months) comparing patients (n=95) with renal dysfunction (estimated glomerular filtration rate 45–60 ml/min/1.73 m2) and without renal dysfunction (estimated glomerular filtration rate >60 ml/min/1.73 m2). Presence of mild-to-moderate renal dysfunction, in 5-year follow-up was associated with higher all-cause mortality (33.3% vs 12.2%, HR=3.16, 95%CI [1.18, 8.51], P=0.016) in multivariate analyses adjusted for age and sex. Log-rank P=0.016.
Figure 2. Kaplan-Meier curve for overall survival (months) comparing patients (n=95) with renal dysfunction (estimated glomerular filtration rate 45–60 ml/min/1.73 m2) and without renal dysfunction (estimated glomerular filtration rate >60 ml/min/1.73 m2). Presence of mild-to-moderate renal dysfunction, in 5-year follow-up was associated with higher all-cause mortality (33.3% vs 12.2%, HR=3.16, 95%CI [1.18, 8.51], P=0.016) in multivariate analyses adjusted for age and sex. Log-rank P=0.016. 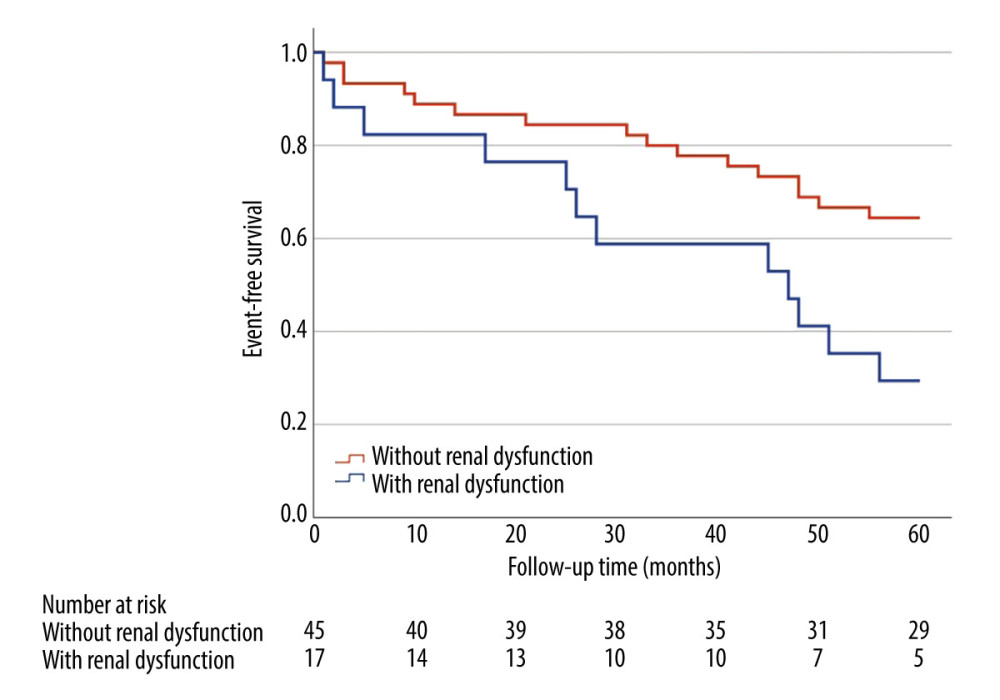 Figure 3. Kaplan-Meier curve for major adverse cardiovascular events (months) comparing patients (n=65) with renal dysfunction (estimated glomerular filtration rate 45–60 ml/min/1.73 m2) and without renal dysfunction (estimated glomerular filtration rate >60 ml/min/1.73 m2). Major adverse cardiovascular events in 5-year follow-up occurred in 70.6% of patients with mild-to-moderate renal dysfunction compared to 36.6% of patients without renal dysfunction (HR=2.54, 95% CI: 1.20–5.38, P=0.011) in a multivariate analysis adjusted for age and sex. Log-rank P=0.011.
Figure 3. Kaplan-Meier curve for major adverse cardiovascular events (months) comparing patients (n=65) with renal dysfunction (estimated glomerular filtration rate 45–60 ml/min/1.73 m2) and without renal dysfunction (estimated glomerular filtration rate >60 ml/min/1.73 m2). Major adverse cardiovascular events in 5-year follow-up occurred in 70.6% of patients with mild-to-moderate renal dysfunction compared to 36.6% of patients without renal dysfunction (HR=2.54, 95% CI: 1.20–5.38, P=0.011) in a multivariate analysis adjusted for age and sex. Log-rank P=0.011. Tables
Table 1. Baseline characteristics. Table 2. Dominant plaque categories at minimal lumen area site in optical coherence tomography.
Table 2. Dominant plaque categories at minimal lumen area site in optical coherence tomography.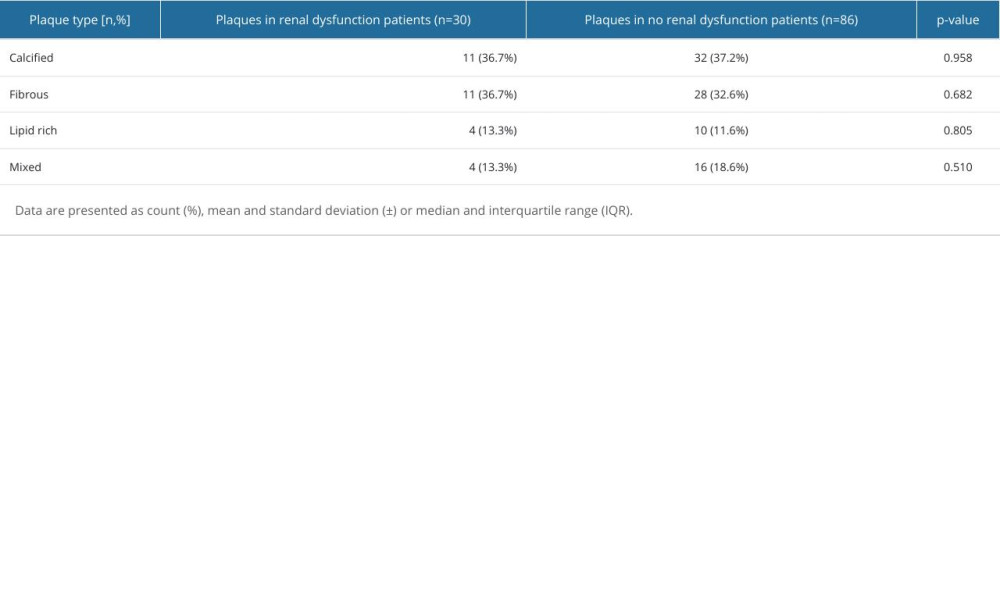 Table 3. Lesion characteristic by optical coherence tomography.
Table 3. Lesion characteristic by optical coherence tomography.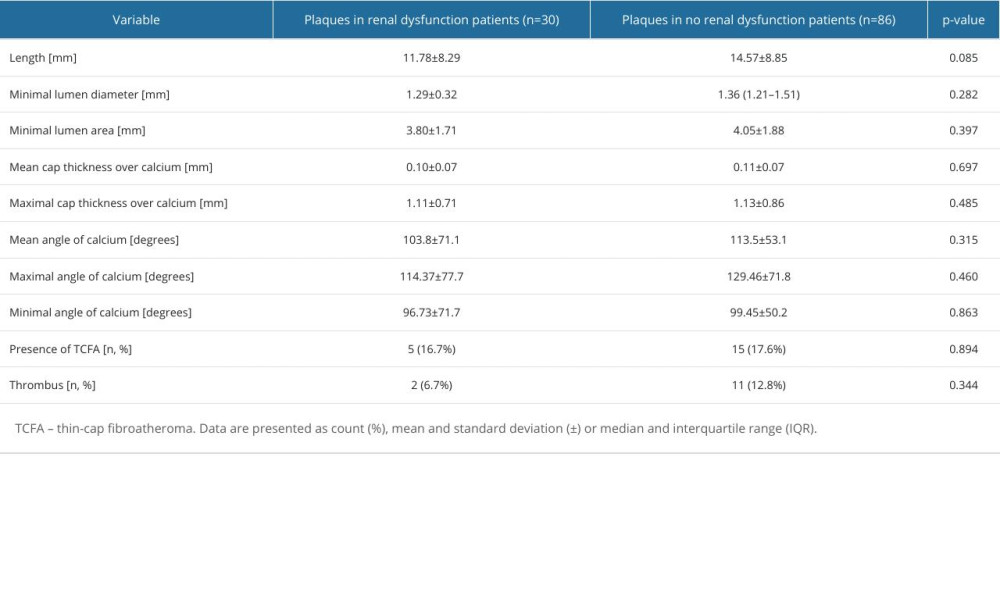 Table 4. Plaque characteristics by intravascular ultrasound.
Table 4. Plaque characteristics by intravascular ultrasound.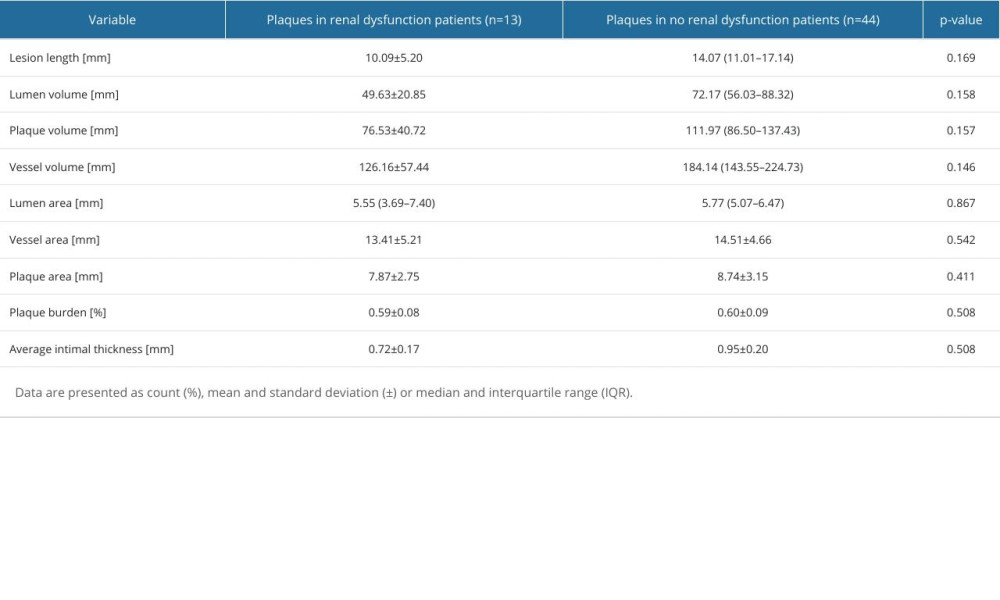 Table 5. Frequency of major adverse cardiovascular events.
Table 5. Frequency of major adverse cardiovascular events.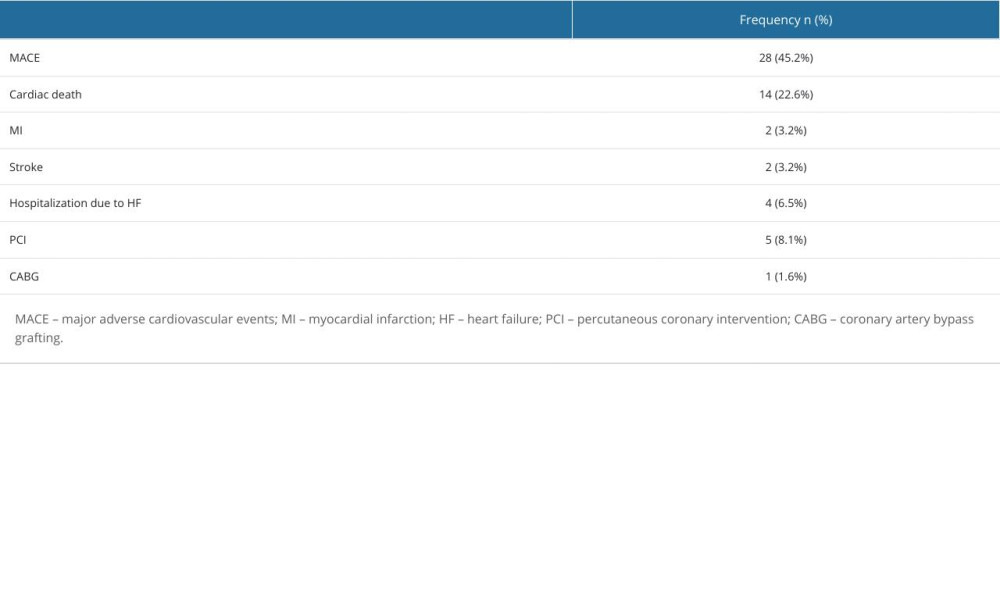
References
1. Go AS, Chertow GM, Fan D, Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization: N Engl J Med, 2004; 351(13); 1296-305
2. Naito R, Miyauchi K, Coronary artery disease and type 2 diabetes mellitus: Int Heart J, 2017; 58(4); 475-80
3. Lewington S, Clarke R, Qizilbash NProspective Studies Collaboration, Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies: Lancet, 2002; 360(9349); 1903-13
4. Manjunath G, Tighiouart H, Ibrahim H, Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community: J Am Coll Cardiol, 2003; 41(1); 47-55
5. Shaw JA, Andrianopoulos N, Duffy S, Renal impairment is an independent predictor of adverse events post coronary intervention in patients with and without drug-eluting stents: Cardiovasc Revasc Med, 2008; 9(4); 218-23
6. Goodman WG, Goldin J, Kuizon BD, Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis: N Engl J Med, 2000; 342(20); 1478-83
7. Gruberg L, Rai P, Mintz GS, Impact of renal function on coronary plaque morphology and morphometry in patients with chronic renal insufficiency as determined by intravascular ultrasound volumetric analysis: Am J Cardiol, 2005; 96(7); 892-96
8. Tobis J, Azarbal B, Slavin L, Assessment of intermediate severity coronary lesions in the catheterization laboratory: J Am Coll Cardiol, 2007; 49(8); 839-48
9. Kennedy MW, Fabris E, Ijsselmuiden AJ, Combined optical coherence tomography morphologic and fractional flow reserve hemodynamic assessment of non-culprit lesions to better predict adverse event outcomes in diabetes mellitus patients: COMBINE (OCT-FFR) prospective study. Rationale and design: Cardiovasc Diabetol, 2016; 15(1); 144
10. De Franco AC, Nissen SE, Coronary intravascular ultrasound: Implications for understanding the development and potential regression of atherosclerosis: Am J Cardiol, 2001; 88(10A); 7M-20M
11. Otake H, Optical coherence tomography-guided percutaneous coronary intervention: Evidence and clinical trials: Interv Cardiol Clin, 2023; 12(2); 225-36
12. Xaplanteris P, Fournier S, Pijls NHJ, Five-year outcomes with PCI guided by fractional flow reserve: N Engl J Med, 2018; 379(3); 250-59
13. Tonino PA, De Bruyne B, Pijls NH, Fractional flow reserve versus angiography for guiding percutaneous coronary intervention: N Engl J Med, 2009; 360(3); 213-24
14. Chin CY, Matsumura M, Maehara A, Coronary plaque characteristics in hemodialysis-dependent patients as assessed by optical coherence tomography: Am J Cardiol, 2017; 119(9); 1313-19
15. Kato K, Yonetsu T, Jia H, Nonculprit coronary plaque characteristics of chronic kidney disease: Circ Cardiovasc Imaging, 2013; 6(3); 448-56
16. Okamura A, Okura H, Iwai S, Incidence and prognostic impact of the calcified nodule in coronary artery disease patients with end-stage renal disease on dialysis: Heart Vessels, 2022; 37(10); 1662-68
17. Kono K, Fujii H, Miyoshi N, Coronary plaque morphology using virtual histology-intravascular ultrasound analysis in hemodialysis patients: Ther Apher Dial, 2011; 15(1); 44-50
18. Miyagi M, Ishii H, Murakami R, Impact of renal function on coronary plaque composition: Nephrol Dial Transplant, 2010; 25(1); 175-81
19. Varela AM, Taniguchi E, Stadler N, Use of intravascular ultrasonography for the characterization of coronary artery disease in patients with chronic kidney disease: J Bras Nefrol, 2011; 33(4); 413-21
20. Lawton JS, Tamis-Holland JE, Bangalore S, 2021 ACC/AHA/SCAI Guideline for coronary artery revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines: Circulation, 2022; 145(3); e18-e114
21. Tebaldi M, Biscaglia S, Fineschi M, Fractional flow reserve evaluation and chronic kidney disease: Analysis from a Multicenter Italian Registry (the FREAK Study): Catheter Cardiovasc Interv, 2016; 88(4); 555-62
22. Collet JP, Thiele H, Barbato E, 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Eur Heart J, 2021; 42(14); 1289-367
23. Ibánez B, James S, Agewall S, 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: Rev Esp Cardiol (English ed), 2017; 70(12); 1082
24. Knuuti J, Wijns W, Saraste A, 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: Eur Heart J, 2020; 41(3); 407-77
25. Ali ZA, Karimi Galougahi K, Mintz GS, Intracoronary optical coherence tomography: State of the art and future directions: EuroIntervention, 2021; 17(2); e105-e23
26. Ali ZA, Maehara A, Genereux P, Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI) : A randomised controlled trial: Lancet, 2016; 388(10060); 2618-28
27. Collet C, De Bruyne B, FFR or OCT or FFR and OCT: JACC Cardiovasc Interv, 2020; 13(1); 59-61
28. Kedhi E, Berta B, Roleder T, Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: The COMBINE OCT-FFR trial: Eur Heart J, 2021; 42(45); 4671-79
29. Legutko J, Bryniarski KL, Kaluza GL, Intracoronary imaging of vulnerable plaque-from clinical research to everyday practice: J Clin Med, 2022; 11(22); 6639
30. Roleder-Dylewska M, Gasior P, Hommels TM, Morphological characteristics of lesions with thin cap fibroatheroma-a substudy from the COMBINE (OCT-FFR) trial: Eur Heart J Cardiovasc Imaging, 2023; 24(5); 687-93
31. Yu W, Huang J, Jia D, Diagnostic accuracy of intracoronary optical coherence tomography-derived fractional flow reserve for assessment of coronary stenosis severity: EuroIntervention, 2019; 15(2); 189-97
32. Ziedses des Plantes AC, Scoccia A, Intravascular imaging-derived physiology-basic principles and clinical application: Cardiol Clin, 2024; 42(1); 89-100
33. Neupane S, Singh H, Edla S, Meta-analysis of fractional flow reserve guided complete revascularization versus infarct related artery only revascularization in patients with ST-elevation myocardial infarction and multivessel coronary artery disease: Coron Artery Dis, 2019; 30(6); 393-97
34. Abo-Aly M, Lolay G, Adams C, Comparison of intracoronary versus intravenous adenosine-induced maximal hyperemia for fractional flow reserve measurement: A systematic review and meta-analysis: Catheter Cardiovasc Interv, 2019; 94(5); 714-21
35. Whayne TF, Sousa MJ, Abdel-Latif A, Use and value of fractional flow reserve in coronary arteriography: Angiology, 2020; 71(1); 5-9
36. Ali ZA, Karimi Galougahi K, Maehara A, Outcomes of optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation: One-year results from the ILUMIEN III: OPTIMIZE PCI trial: EuroIntervention, 2021; 16(13); 1085-91
37. Baruś P, Modrzewski J, Gumiężna K, Comparative appraisal of intravascular ultrasound and optical coherence tomography in invasive coronary imaging: 2022 update: J Clin Med, 2022; 11(14); 4055
38. Johnson TW, Raber L, di Mario C, Clinical use of intracoronary imaging. Part 2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions: Eur Heart J, 2019; 40(31); 2566-84
39. Kim N, Lee JH, Jang SY, Intravascular modality-guided versus angiography-guided percutaneous coronary intervention in acute myocardial infarction: Catheter Cardiovasc Interv, 2020; 95(4); 696-703
40. Neumann FJ, Sousa-Uva M, Ahlsson A, 2018 ESC/EACTS Guidelines on myocardial revascularization: Eur Heart J, 2019; 40(2); 87-165
41. Oosterveer TTM, van der Meer SM, Scherptong RWC, Jukema JW, Optical coherence tomography: Current applications for the assessment of coronary artery disease and guidance of percutaneous coronary interventions: Cardiol Ther, 2020; 9(2); 307-21
42. Pawlowski T, Legutko J, Kochman J, Clinical use of intracoronary imaging modalities in Poland. Expert opinion of the Association of Cardiovascular Interventions of the Polish Cardiac Society: Kardiol Pol, 2022; 80(4); 509-19
43. Maehara A, Matsumura M, Ali ZA, IVUS-guided versus OCT-guided coronary stent implantation: A critical appraisal: JACC Cardiovasc Imaging, 2017; 10(12); 1487-503
44. Shlofmitz E, Jeremias A, Parviz Y, External elastic lamina vs. luminal diameter measurement for determining stent diameter by optical coherence tomography: An ILUMIEN III substudy: Eur Heart J Cardiovasc Imaging, 2021; 22(7); 753-59
45. Serruys PW, Katagiri Y, Sotomi Y, Arterial remodeling after bioresorbable scaffolds and metallic stents: J Am Coll Cardiol, 2017; 70(1); 60-74
46. Bourantas CV, Garcia-Garcia HM, Farooq V, Clinical and angiographic characteristics of patients likely to have vulnerable plaques: Analysis from the PROSPECT study: JACC Cardiovasc Imaging, 2013; 6(12); 1263-72
47. Erlinge D, Maehara A, Ben-Yehuda O, Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): A prospective natural history study: Lancet, 2021; 397(10278); 985-95
48. Fabris E, Berta B, Roleder T, Thin-cap fibroatheroma rather than any lipid plaques increases the risk of cardiovascular events in diabetic patients: Insights from the COMBINE OCT-FFR Trial: Circ Cardiovasc Interv, 2022; 15(5); e011728
49. Gatto L, Alfonso F, Paoletti G, Relationship betweeen the amount and location of macrophages and clinical outcome: subanalysis of the CLIMA-study: Int J Cardiol, 2022; 346; 8-12
50. Ochijewicz D, Tomaniak M, Koltowski L, Intravascular imaging of coronary artery disease: Recent progress and future directions: J Cardiovasc Med (Hagerstown), 2017; 18(10); 733-41
51. Tomaniak M, Katagiri Y, Modolo R, Vulnerable plaques and patients: State-of-the-art: Eur Heart J, 2020; 41(31); 2997-3004
52. Ueki Y, Yamaji K, Losdat S, Discordance in the diagnostic assessment of vulnerable plaques between radiofrequency intravascular ultrasound versus optical coherence tomography among patients with acute myocardial infarction: insights from the IBIS-4 study: Int J Cardiovasc Imaging, 2021; 37(10); 2839-47
53. Zhang W, Mintz GS, Cao Y, Clinical determinants of coronary artery disease burden and vulnerability using optical coherence tomography co-registered with intravascular ultrasound: Coron Artery Dis, 2022; 33(2); 114-24
54. Tomaniak M, Ochijewicz D, Koltowski L, OCT-derived plaque morphology and FFR-determined hemodynamic relevance in intermediate coronary stenoses: J Clin Med, 2021; 10(11); 2379
55. Barus P, Piasecki A, Gumiezna K, Multimodality OCT, IVUS and FFR evaluation of coronary intermediate grade lesions in women vs. men: Front Cardiovasc Med, 2023; 10; 1021023
56. Prati F, Cera M, Ramazzotti V, Imola F, Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios: EuroIntervention, 2007; 3(3); 365-70
57. Prati F, Ramazzotti V, Fernandez B, Albertucci M, The non-occlusive modality of optical coherence tomography image acquisition: A new concept for wide clinical application: G Ital Cardiol (Rome), 2009; 10(10); 644-49 [in Italian]
58. Prati F, Guagliumi G, Mintz GS, Expert review document part 2: Methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures: Eur Heart J, 2012; 33(20); 2513-20
59. Prati F, Regar E, Mintz GS, Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis: Eur Heart J, 2010; 31(4); 401-15
60. Raber L, Mintz GS, Koskinas KC, Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions: Eu Heart J, 2018; 39(35); 3281-300
61. Tearney GJ, Regar E, Akasaka T, Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation: J Am Coll Cardiol, 2012; 59(12); 1058-72
62. Kini AS, Vengrenyuk Y, Yoshimura T, Fibrous cap thickness by optical coherence tomography in vivo: J Am Coll Cardiol, 2017; 69(6); 644-57
63. Blaziak M, Urban S, Jura M, Kuliczkowski W, Fractional flow reserve-guided treatment in coronary artery disease: Clinical practice: Adv Clin Exp Med, 2021; 30(10); 1075-84
64. Götberg M, Christiansen EH, Gudmundsdottir IJ, Instantaneous wave-free ratio versus fractional flow reserve to guide PCI: N Engl J Med, 2017; 376(19); 1813-23
65. Pijls NH, Tanaka N, Fearon WF, Functional assessment of coronary stenoses: Can we live without it?: Eur Heart J, 2013; 34(18); 1335-44
66. Sels JW, Tonino PA, Siebert U, Fractional flow reserve in unstable angina and non-ST-segment elevation myocardial infarction experience from the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) study: JACC Cardiovascular interventions, 2011; 4(11); 1183-89
67. Balocco S, Gatta C, Ciompi F, Standardized evaluation methodology and reference database for evaluating IVUS image segmentation: Comput Med Imaging Graph, 2014; 38(2); 70-90
68. Schoenhagen P, Nissen SE, Assessing coronary plaque burden and plaque vulnerability: Atherosclerosis imaging with IVUS and emerging noninvasive modalities: Am Heart Hosp J, 2003; 1(2); 164-69
69. Chade AR, Brosh D, Higano ST, Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease: Kidney Int, 2006; 69(2); 266-71
70. Yilmaz MB, Yalta K, Coronary flow slows as renal function worsens: Clin Cardiol, 2009; 32(5); 278-82
71. Sugiyama T, Kimura S, Ohtani H, Impact of chronic kidney disease stages on atherosclerotic plaque components on optical coherence tomography in patients with coronary artery disease: Cardiovasc Interv Ther, 2017; 32(3); 216-24
72. Baber U, Stone GW, Weisz G, Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes: JACC Cardiovasc Imaging, 2012; 5(3 Suppl); S53-61
73. Nakajima A, Araki M, Kurihara O, Predictors for rapid progression of coronary calcification: An optical coherence tomography study: J Am Heart Assoc, 2021; 10(3); e019235
74. Burzotta F, Leone AM, Aurigemma C, Fractional flow reserve or optical coherence tomography to guide management of angiographically intermediate coronary stenosis: A single-center trial: JACC Cardiovasc Interv, 2020; 13(1); 49-58
Figures
 Figure 1. Examples of plaque types in optical coherence tomography. (A) Healthy vessel (normal three-layer artery wall visible), (B) Fibrous plaques (homogenous signal, high backscattering), (C) Calcified plaque (signal-poor heterogeneous region with sharply delineated border), (D) lipid-rich plaque (inhomogeneous signal-poor region with diffused borders). Plaques are marked with an *.
Figure 1. Examples of plaque types in optical coherence tomography. (A) Healthy vessel (normal three-layer artery wall visible), (B) Fibrous plaques (homogenous signal, high backscattering), (C) Calcified plaque (signal-poor heterogeneous region with sharply delineated border), (D) lipid-rich plaque (inhomogeneous signal-poor region with diffused borders). Plaques are marked with an *.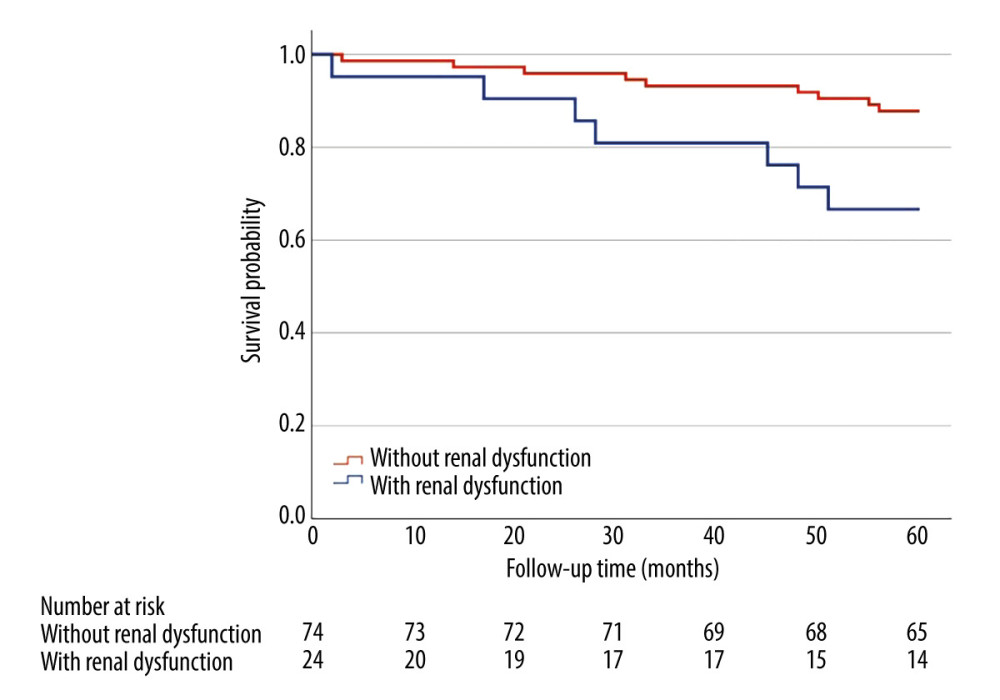 Figure 2. Kaplan-Meier curve for overall survival (months) comparing patients (n=95) with renal dysfunction (estimated glomerular filtration rate 45–60 ml/min/1.73 m2) and without renal dysfunction (estimated glomerular filtration rate >60 ml/min/1.73 m2). Presence of mild-to-moderate renal dysfunction, in 5-year follow-up was associated with higher all-cause mortality (33.3% vs 12.2%, HR=3.16, 95%CI [1.18, 8.51], P=0.016) in multivariate analyses adjusted for age and sex. Log-rank P=0.016.
Figure 2. Kaplan-Meier curve for overall survival (months) comparing patients (n=95) with renal dysfunction (estimated glomerular filtration rate 45–60 ml/min/1.73 m2) and without renal dysfunction (estimated glomerular filtration rate >60 ml/min/1.73 m2). Presence of mild-to-moderate renal dysfunction, in 5-year follow-up was associated with higher all-cause mortality (33.3% vs 12.2%, HR=3.16, 95%CI [1.18, 8.51], P=0.016) in multivariate analyses adjusted for age and sex. Log-rank P=0.016. Figure 3. Kaplan-Meier curve for major adverse cardiovascular events (months) comparing patients (n=65) with renal dysfunction (estimated glomerular filtration rate 45–60 ml/min/1.73 m2) and without renal dysfunction (estimated glomerular filtration rate >60 ml/min/1.73 m2). Major adverse cardiovascular events in 5-year follow-up occurred in 70.6% of patients with mild-to-moderate renal dysfunction compared to 36.6% of patients without renal dysfunction (HR=2.54, 95% CI: 1.20–5.38, P=0.011) in a multivariate analysis adjusted for age and sex. Log-rank P=0.011.
Figure 3. Kaplan-Meier curve for major adverse cardiovascular events (months) comparing patients (n=65) with renal dysfunction (estimated glomerular filtration rate 45–60 ml/min/1.73 m2) and without renal dysfunction (estimated glomerular filtration rate >60 ml/min/1.73 m2). Major adverse cardiovascular events in 5-year follow-up occurred in 70.6% of patients with mild-to-moderate renal dysfunction compared to 36.6% of patients without renal dysfunction (HR=2.54, 95% CI: 1.20–5.38, P=0.011) in a multivariate analysis adjusted for age and sex. Log-rank P=0.011. Tables
 Table 1. Baseline characteristics.
Table 1. Baseline characteristics. Table 2. Dominant plaque categories at minimal lumen area site in optical coherence tomography.
Table 2. Dominant plaque categories at minimal lumen area site in optical coherence tomography. Table 3. Lesion characteristic by optical coherence tomography.
Table 3. Lesion characteristic by optical coherence tomography. Table 4. Plaque characteristics by intravascular ultrasound.
Table 4. Plaque characteristics by intravascular ultrasound. Table 5. Frequency of major adverse cardiovascular events.
Table 5. Frequency of major adverse cardiovascular events. Table 1. Baseline characteristics.
Table 1. Baseline characteristics. Table 2. Dominant plaque categories at minimal lumen area site in optical coherence tomography.
Table 2. Dominant plaque categories at minimal lumen area site in optical coherence tomography. Table 3. Lesion characteristic by optical coherence tomography.
Table 3. Lesion characteristic by optical coherence tomography. Table 4. Plaque characteristics by intravascular ultrasound.
Table 4. Plaque characteristics by intravascular ultrasound. Table 5. Frequency of major adverse cardiovascular events.
Table 5. Frequency of major adverse cardiovascular events. In Press
07 May 2024 : Clinical Research
Treatment of AVN-Induced Proximal Pole Scaphoid Nonunion Using a Fifth and Fourth Extensor Compartmental Ar...Med Sci Monit In Press; DOI: 10.12659/MSM.944553
16 Mar 2024 : Clinical Research
Diagnostic Efficiency of ACR-TIRADS Score for Differentiating Benign and Malignant Thyroid Nodules of Vario...Med Sci Monit In Press; DOI: 10.12659/MSM.943228
08 May 2024 : Clinical Research
Effect of Individualized PEEP Guided by Driving Pressure on Diaphragm Function in Patients Undergoing Lapar...Med Sci Monit In Press; DOI: 10.12659/MSM.944022
21 Mar 2024 : Clinical Research
Impact of Serum Vitamin D, B6, and B12 and Cognitive Functions on Quality of Life in Peri- and Postmenopaus...Med Sci Monit In Press; DOI: 10.12659/MSM.943249
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








