21 April 2024: Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, and High-Income Countries: A Systematic Review
Abdul Khairul Rizki PurbaDOI: 10.12659/MSM.943863
Med Sci Monit 2024; 30:e943863
Abstract
BACKGROUND: Economic evaluation of the testing strategies to control transmission and monitor the severity of COVID-19 after the pandemic is essential. This study aimed to review the economic evaluation of COVID-19 tests and to construct a model with outcomes in terms of cost and test acceptability for surveillance in the post-pandemic period in low-income, middle-income, and high-income countries.
MATERIAL AND METHODS: We performed the systematic review following PRISMA guidelines through MEDLINE and EMBASE databases. We included the relevant studies that reported the economic evaluation of COVID-19 tests for surveillance. Also, we input current probability, sensitivity, and specificity for COVID-19 surveillance in the post-pandemic period.
RESULTS: A total of 104 articles met the eligibility criteria, and 8 articles were reviewed and assessed for quality. The specificity and sensitivity of COVID-19 screening tests were reported as 80% to 90% and 40% to 90%, respectively. The target population presented a mortality rate between 0.2% and 19.2% in the post-pandemic period. The implementation model of COVID-19 screening tests for surveillance with a cost mean for molecular and antigen tests was US$ 46.64 (min-max US $0.25-$105.39) and US $6.15 (min-max US $2-$10), respectively.
CONCLUSIONS: For the allocation budget for the COVID-19 surveillance test, it is essential to consider the incidence and mortality of the post-pandemic period in low-income, middle-income, and high-income countries. A robust method to evaluate outcomes is needed to prevent increasing COVID-19 incidents earlier.
Keywords: Cost of Illness, Epidemiology, Mass Screening, SARS-CoV-2, Mortality
Introduction
The burden of COVID-19 encompasses various aspects, including the direct impacts on health, mortality, and broader societal implications. The direct burden of the COVID-19 pandemic has varied across countries, with significant morbidity and mortality globally [1,2]. The pandemic has also led to challenges in mental health, particularly for children and adolescents, due to factors such as anxiety, social isolation, and economic distress [3]. Furthermore, the pandemic has raised ethical, legal, and social issues, such as triage, contact tracing, and quarantine measures, which have significant implications for public policy and healthcare systems. The burden of COVID-19 is far greater in developing countries than in high-income countries, reflecting elevated transmission and limited healthcare access in developing countries [1]. Notably, the evidence underscores the need for comprehensive strategies to address the multifaceted burden of the pandemic on a global scale.
The official count of deaths attributed to COVID-19 was slightly more than 5.4 million around December 2021. However, the death number is likely higher, due to under-reporting and differences in counting methods across countries [4]. A high mortality rate can be found in countries such as Peru, which had the highest number of COVID-19 deaths per capita. Other countries with high mortality rates include Russia, Mexico, Brazil, Indonesia, and Pakistan. The global all-age rate of excess mortality due to the COVID-19 pandemic was 120.3 deaths (113.1–129.3) per 100 000 population. Excess mortality rates exceeded 300 deaths per 100 000 in 21 countries [5].
Various organizations and governments have developed COVID-19 testing strategies to control the transmission of the virus and monitor its severity in the post-pandemic period. These strategies aim to achieve universal testing of all people with COVID-19 symptoms, which is resource-intensive. The European Centre for Disease Prevention and Control has proposed 5 main objectives for testing, including controlling transmission and reliably monitoring severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission rates and severity [6]. The Royal College of Pathologists has also outlined a national testing strategy, emphasizing 7 principles underpinning any diagnostic assessment [7]. There are 3 main types of COVID-19 tests: molecular, antigen, and antibody. Molecular tests, such as polymerase chain reaction (PCR) and other nucleic acid amplification tests, detect the genetic material ribonucleic acid from the virus and are considered the criterion standard for COVID-19 tests [8]. The accuracy of reported numbers of COVID-19 cases and deaths can be affected by factors such as the lack of testing, asymptomatic distribution, and differences in testing methods across countries. Understanding these limitations is essential for implementing effective public health measures and informing policy decisions [9]. The delayed diagnosis of COVID-19 can significantly impact patients’ quality of life. Evidence shows some patients experience prolonged symptoms after COVID-19 infection (long COVID), reducing their quality of life, compared with that healthy individuals [10]. Early diagnosis is crucial for accessing supportive measures to maximize patients’ quality of life. However, delays in COVID-19 testing results can hinder the response and management of the disease [11]. It is essential to address the limitations of COVID-19 testing and case data to ensure evidence-informed health policy and practice. Molecular tests, such as PCR, are widely used to diagnose COVID-19, due to their high sensitivity, but delayed or inaccurate results can impact patient care and quality of life [8]. Therefore, improving testing capacity and reducing delays in diagnostic results are essential for mitigating the impact of COVID-19 on the quality of life of affected individuals. This systematic review aims to evaluate the cost analysis of screening tests for COVID-19 and infection surveillance in the post-pandemic period in low-income, middle-income (MIC), and high-income countries.
Material and Methods
STUDY SELECTION:
We included all studies that analyzed the cost of COVID-19 testing for surveillance with model-based or trial-based economic evaluation designs performed in low-income countries, lower-middle-income countries, upper-middle-income countries, and high-income countries. The scope of the study was economic evaluation, including cost minimization, cost-effectiveness analysis, cost-benefit analysis, and cost utility. We excluded non-research studies, such as book chapters, reports, conference proceedings, and review articles. Authors AKRP, ANR, SH, RP, and AN searched the articles using the Boolean operator (OR/AND) with PICO strategy [16], where the problem (P) was a targeted population with low or high COVID-19 incidence; intervention (I) was screening testing; comparison (C) was any tests; and outcomes (O) were cost and effectiveness.
DATA EXTRACTION:
Two authors (AKRP and AN) independently extracted data on the author, country, year of publication, age, number of subjects, intervention, study design model, time horizon, duration, cost, cost perspective, discount rate, and all outcomes, including disability-adjusted life years or quality-adjusted life years or life year gain. We collected all relevant outcomes, including risk ratio, odds ratio, mean difference, statistical significance, statistical dispersion, standard deviation (SD), and range. All costs were converted into USD following the year’s currency when the included study was conducted. The currency was set 1 year prior to the publication year if the study year was not mentioned. In addition, we reported the incremental cost-effectiveness, sensitivity, and specificity of screening tests.
COST ANALYSIS AND DATA SYNTHESIS:
All types of economic evaluations were included in the cost analysis, such as cost minimization analysis, cost-benefit analysis, cost-effectiveness analysis, and cost-utility analysis [17]. In addition, we considered partial economic evaluations that did not include the consequences of therapeutic strategies and costs [18,19]. In cases of unclear classification, the 2 researchers discussed differences until an agreement was reached. Notably, we developed a model for the cost of COVID-19 surveillance in the post-pandemic period. We included direct healthcare costs, such as hospitalization, medication, chemotherapy, laboratory tests, screening tests, radiotherapy, Intensive Care Unit (ICU) admission, emergency room admission, outpatient healthcare cost, death averted cost, isolation of positive cases, human resources, physical therapist, imaging diagnosis, and medical follow-up; direct non-health costs, such as social service costs and transportation; and indirect costs, such as lost productivity and premature death. We applied a 3% discount rate for both cost and effectiveness outcomes. We assumed no advanced infection after COVID-19, no social subsidies for infection daily costs, and no additional costs for non-tested populations. Also, the study assumption included similar immunity and vaccination status among the targeted population. In addition, we categorized the study background into high-income countries, upper-middle-income countries, lower-middle-income countries, and low-income countries. We input the probability and costs into a model using TreeAge Pro.
STATISTICAL ANALYSIS:
The collected data from selected studies were presented as a descriptive report following the PRISMA guideline. Probability populations targeted in COVID-19 and severe COVID-19 screening and mortality were presented based on the categories of high-income countries, upper-middle-income countries, lower-middle-income countries, and low-income countries. We calculated specificity and sensitivity (molecular test and antigen test) and costs of diagnostic test mean (min–max, molecular test and antigen test), personal protective equipment, human resources, hospitalization cost, ICU admission cost, outpatient healthcare cost, productivity loss, death averted cost, and isolation of positive cases.
Results
SELECTED STUDIES:
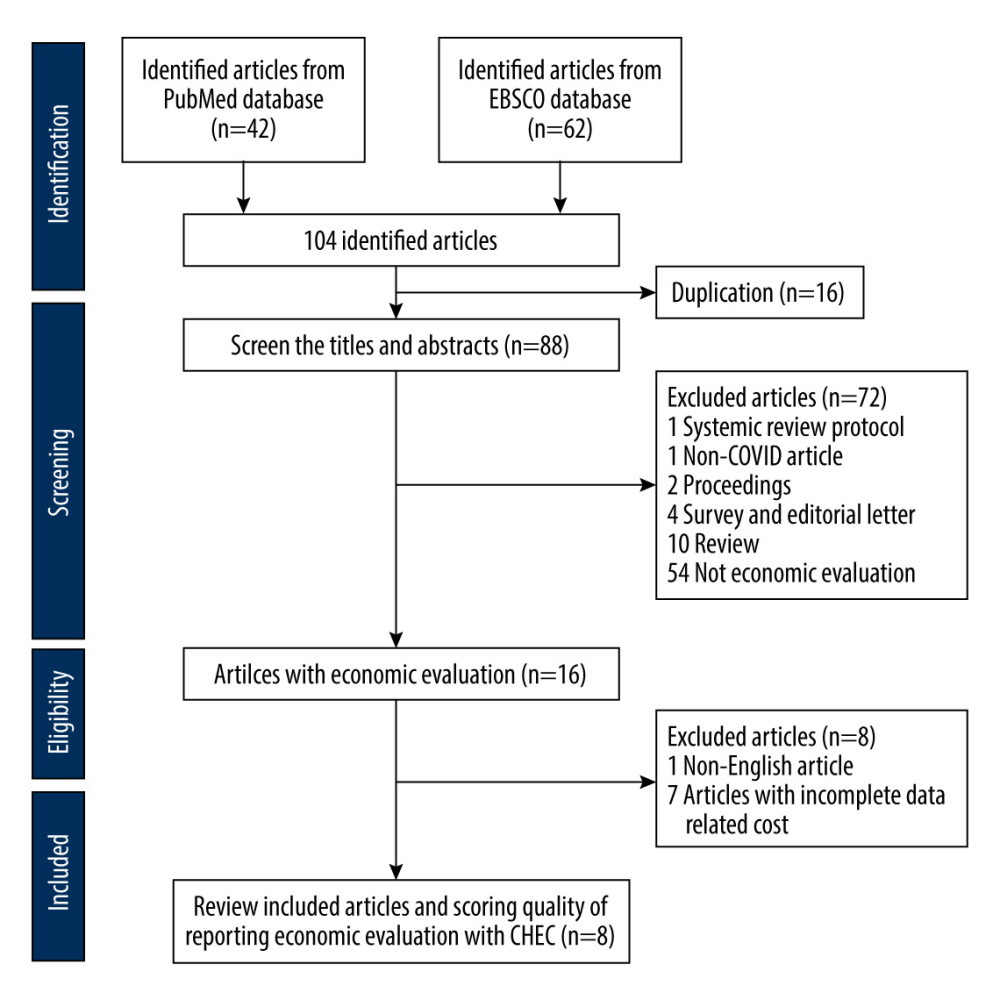
We identified 104 articles from PubMed and EBSCO databases. After removing duplications and screening the abstracts according to inclusion and exclusion criteria, we assessed 8 articles for publishing quality using CHEC (Figure 1). Among the selected articles, 5 articles were performed in the United States [20–24], and the others were conducted in the United Kingdom [25], Italy [26], and China [27]. The types of tests encompassed molecular and antigen tests. Three studies presented specificity, with a range of 80% to 99%, and 4 studies reported sensitivity of the screening tests from 40% to 99% (Table 1).
COST EVALUATION:
We extracted the economic outcomes, including the intervention model, relevant costs, health-related quality of life, and incremental costs (Table 2). Notably, we input the extracted data into a model for cost analysis of COVID-19 testing in high-income countries, upper-middle-income countries, lower-middle-income countries, and low-income countries in the post-pandemic era, with an incidence of 53.8%, 33.5%, 12.4%, and 0.30%, and low mortality of 0.003%, 0.002%, 0.010%, and 0.192%, respectively. The costs for molecular and antigen tests were US $46.64 (min–max: US $0.25–$105.39) and US $6.15 (min–max: US $2–$10), respectively. We inputted all relevant hospitalization costs of US$ 2.77 for personal protective equipment, US $5287 for human resources, US $9677 for ICU admission, and US $200 for productivity loss. Outpatients would be considered with accounts of US$ 46 687 for healthcare costs and US $674 for isolation (Table 3). Furthermore, the implementation of COVID-19 testing for surveillance in low-income countries would need the lowest cost (mean: US $10 718.84; SD: ±179.12) followed by lower-middle-income countries (mean: $6672.37; SD: ±150.56), upper-middle-income countries (mean: US $2472.09; SD: ±94.07), and high-income countries (mean: US $59.54; SD: ±15.88).
Discussion
The study findings present evidence of economic evaluation in using screening tests for COVID-19 surveillance. The data are primarily from the United States, United Kingdom, Italy, and China, with high-income and upper-middle-income country categories. The value of the systematic review is delivered in the analysis of the COVID-19 tests after the pandemic. Implementing the molecular and antigen tests would be most acceptable for COVID-19 surveillance in low-income countries, followed by lower-middle-income countries, upper-middle-income countries, and high-income countries. This is in line with considering the remaining high incidence in high-income countries, where surveillance is essential to achieve further morbidity, quality-adjusted life years, and mortality outcomes.
The sensitivity of COVID-19 tests, including rapid antigen tests, can vary depending on multiple factors, such as symptoms, specific viral variant being tested, and type of specimen collected [28]. It is essential to consider these factors when interpreting test results and to make decisions based on the testing data. The sensitivity of COVID-19 testing varies depending on the type of test and the specific conditions. Rapid antigen tests have varying sensitivities, and their performance can differ depending on factors such as the presence of symptoms and the specific viral variant being tested [29,30]. In people with signs and symptoms of COVID-19, the sensitivity of antigen tests is highest in the first week of illness, when viral loads are higher [29]. For asymptomatic individuals, the sensitivity of antigen tests is higher when an epidemiological exposure to SARS-CoV-2 is suspected, compared with situations in which COVID-19 testing is widely available to anyone [31]. The sensitivity of current FDA-authorized antigen tests is lower than that of nucleic acid amplification tests [32]. A study comparing healthcare workers collected throat specimens to self-collected nasal specimens found that throat specimens can have higher sensitivity to COVID-19 rapid antigen testing. In comparison, nasal specimens had higher sensitivity for self-collected specimens [33]. Rapid upscaling of in vitro diagnostic assays is crucial for mass screening and testing of high-risk groups during the SARS-CoV-2 and COVID-19 pandemics. As testing needs increase, molecular and serological tests have been developed quickly and on various platforms. Understanding the advantages and disadvantages of these tests is essential for policymakers and healthcare professionals to create effective treatment plans and public health initiatives [34].
The cost of COVID-19 test screening surveillance in high-income countries depends on the type of surveillance system being implemented, the resources required, and the specific context of the country or region. It is essential to consider these factors when planning and implementing surveillance systems to monitor the spread of COVID-19. A study conducted in Germany estimated the economic cost of establishing and implementing 4 active surveillance strategies using experimental data collected within the Cov-Surv-Study trial framework. The study found that the average cost per sample tested for direct testing of individuals and direct testing of households was EUR 63.33 [35]. Mass testing with rapid surveillance tests coupled with strict but relatively short isolation of confirmed cases can be a cost-effective strategy for controlling COVID-19 transmission [21]. Another study conducted in Colombia, including representatives from upper-middle-income countries, assessed the cost-effectiveness of the COVID-19 test, trace, and isolate program, including program costs of time-to-intervention, health services utilization, hospitalization, and mortality rates [36]. Implementation of the cost-effective mass surveillance of COVID-19 in low- and middle-income countries can have challenges and opportunities, and the costs will depend on factors such as resource mobilization and local capacity building.
A study conducted in Massachusetts projected the clinical and economic impact of alternative testing strategies on COVID-19 incidence and mortality. The study found that testing people with any COVID-19-consistent symptoms would be cost-saving, compared with restricting expanding PCR testing to asymptomatic people, which would decrease infections, deaths, and hospitalizations. Universal screening would be cost-effective when paired with monthly retesting in settings where planning is essential for utilizing key limited resources, such as testing and hospital beds [37]. In low- and middle-income countries, swab-based COVID-19 PCR testing costs more than US $200, while saliva-based testing approaches are comparatively less costly [38]. A cost-effectiveness analysis of different COVID-19 screening strategies based on rapid or laboratory-based SARS-CoV-2 antigen testing found that in situations where test sensitivity is of paramount importance, nucleic acid amplification tests are preferred [39]. Low and middle-income countries have been pledged 120 million COVID-19 diagnostic tests, which can provide results in 15 to 30 min, with the price set at US $5 per unit and the expectation that the cost will decrease [40]. In addition, it was not possible to suggest the fast test antibody or serological test of IgM and IgG as a diagnostic aid. A study documented no relationship between positive SARS-CoV PCR results and reactive rapid test results [41].
As an observational study, this study has limitations as a systematic review and economic evaluation model. Notably, the review may be less representative of upper-middle-income countries, and further for the lower-middle-income countries and low-income countries. The quality assessment results using the CHEC scores among selected studies indicated that important economic variables were not reported. Valuing the outcomes of humanistic and cost measurements with rigorous methods is needed. Also, the assumption of the insignificant impact of vaccination and immunity status that may have contributed to the outcomes. Moreover, interpreting the results should be done cautiously, since the pandemic’s baseline data were converted into the post-COVID era.
Conclusions
The surveillance after the COVID-19 pandemic considered the screening tests with the allocation budget according to the current incidence and mortality in high-income countries, upper-middle-income countries, lower-middle-income countries, and low-income countries. High sensitivity and specificity of the tests and immunity status are accounted for in the surveillance. The strategies and modalities should be supported by further evidence, with a robust approach in evaluating outcomes for the earlier prevention of the increasing number of COVID-19 incidents.
Tables
Table 1. Characteristics of selected studies.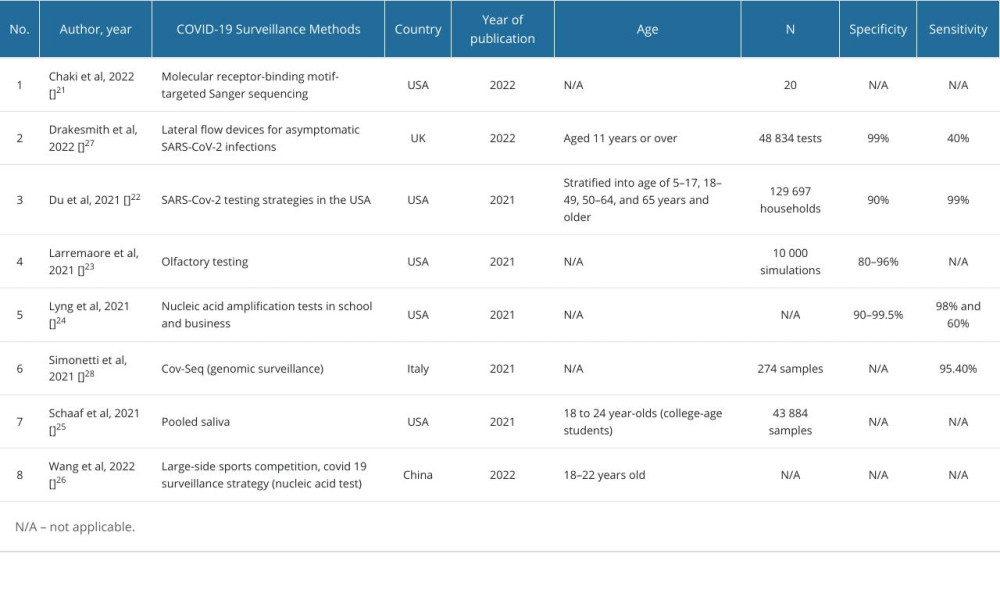 Table 2. Economic evaluation, cost outcome, and health related quality of life.
Table 2. Economic evaluation, cost outcome, and health related quality of life.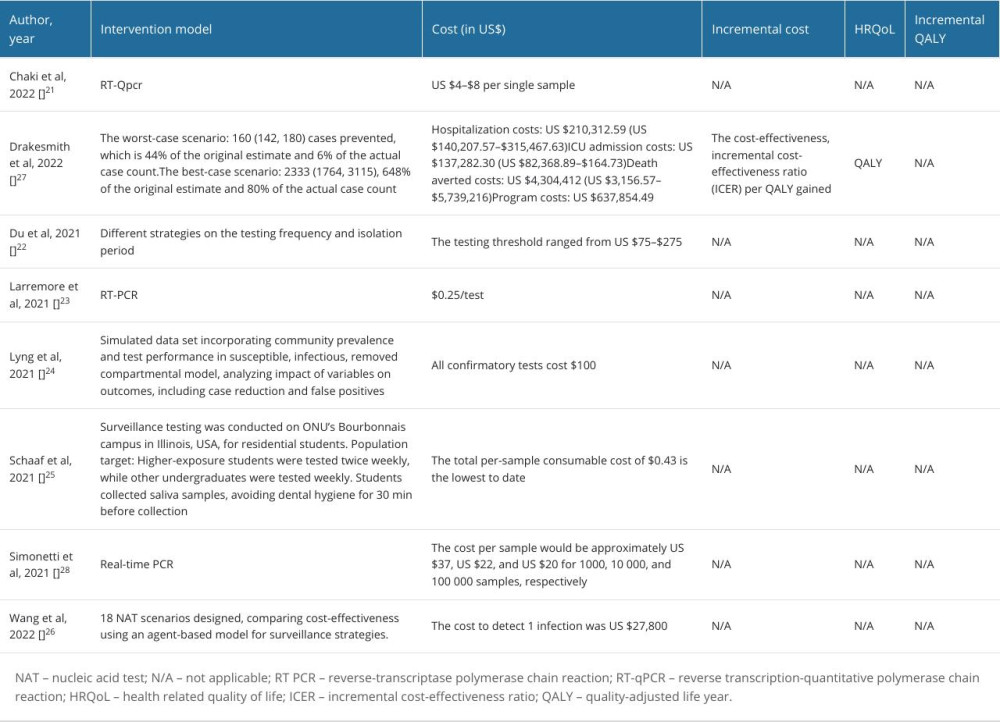 Table 3. Model input for cost analysis of COVID-19 testing in high-income countries (HIC), upper-middle-income countries (UMIC), lower-middle-income countries (LMIC), and low-income countries (LIC) for the post-pandemic era.
Table 3. Model input for cost analysis of COVID-19 testing in high-income countries (HIC), upper-middle-income countries (UMIC), lower-middle-income countries (LMIC), and low-income countries (LIC) for the post-pandemic era.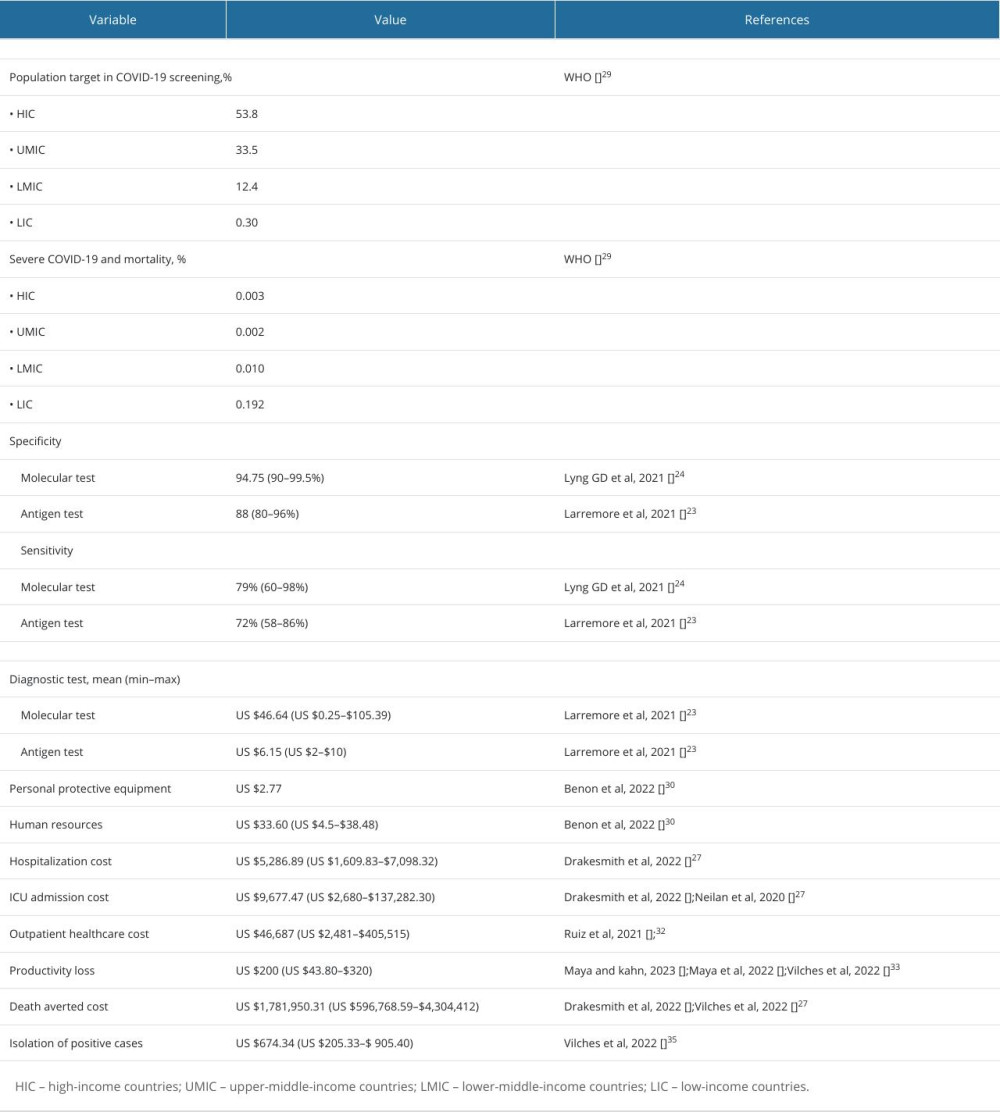
References
1. Pires SM, Wyper GMA, Wengler A, Burden of disease of COVID-19: Strengthening the collaboration for national studies: Front Public Health, 2022; 10; 907012
2. Gebru AA, Birhanu T, Wendimu E, Global burden of COVID-19: Situational analyis and review: Hum Antibodies, 2021; 29(2); 139-48
3. Fegert JM, Vitiello B, Plener PL, Clemens V, Challenges and burden of the Coronavirus 2019 (COVID-19) pandemic for child and adolescent mental health: A narrative review to highlight clinical and research needs in the acute phase and the long return to normality: Child Adolesc Psychiatry Ment Health, 2020; 14; 20
4. Mathieu E, Ritchie H, Rodés-Guirao L: Coronavirus pandemic (COVID-19), 2020 Published online at . Available from: OurWorldInData.org
5. COVID-19 Excess Mortality Collaborators, Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–21: Lancet, 2022; 399(10334); 1513-36 Erratum in: Lancet. 2022;399(10334):1468
6. Suk JE, Pharris A, Beauté J, Public health considerations for transitioning beyond the acute phase of the COVID-19 pandemic in the EU/EEA: Euro Surveill, 2022; 27(17); 2200155
7. Manabe YC, Sharfstein JS, Armstrong K, The need for more and better testing for COVID-19: JAMA, 2020; 324(21); 2153-54
8. Zhang Y, Garner R, Salehi S, Molecular and antigen tests, and sample types for diagnosis of COVID-19: A review: Future Virol, 2022; 17(9); 675-85
9. Alvarez E, Bielska IA, Hopkins S, Limitations of COVID-19 testing and case data for evidence-informed health policy and practice: Health Res Policy Syst, 2023; 21(1); 11
10. Líška D, Liptaková E, Babičová A, What is the quality of life in patients with long COVID compared to a healthy control group?: Front Public Health, 2022; 10; 975992
11. Torres I, Sippy R, Sacoto F, Assessing critical gaps in COVID-19 testing capacity: The case of delayed results in Ecuador: BMC Public Health, 2021; 21(1); 637
12. Purba AKR, Rosyid AN, Handayani S, Cost analysis of screening testing for COVID-19 surveillance: A systematic review, 2022
13. Page MJ, McKenzie JE, Bossuyt PM, The PRISMA 2020 statement: An updated guideline for reporting systematic reviews: BMJ, 2021; 372; n71
14. Bramer WM, de Jonge GB, Rethlefsen ML, A systematic approach to searching: An efficient and complete method to develop literature searches: J Med Libr Assoc, 2018; 106(4); 531-41
15. Liberati A, Altman DG, Tetzlaff J, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration: BMJ, 2009; 339; b2700
16. da Costa Santos CM, de Mattos Pimenta CA, Nobre MR, The PICO strategy for the research question construction and evidence search: Rev Lat Am Enfermagem, 2007; 15(3); 508-11
17. Reeder CE, Overview of pharmacoeconomics and pharmaceutical outcomes evaluations: Am J Health Syst Pharm, 1995; 52(19 Suppl 4); S5-8
18. McLaughlin N, Ong MK, Tabbush V, Contemporary health care economics: An overview: Neurosurg Focus, 2014; 37(5); E2
19. Emmert M, Eijkenaar F, Kemter H, Economic evaluation of pay-for-performance in health care: A systematic review: Eur J Health Econ, 2012; 13(6); 755-67
20. Chaki SP, Kahl-McDonagh MM, Neuman BW, Zuelke KA, Receptor-binding-motif-targeted sanger sequencing: A quick and cost-effective strategy for molecular surveillance of SARS-CoV-2 variants: Microbiol Spectr, 2022; 10(3); e0066522
21. Du Z, Pandey A, Bai Y, Fitzpatrick MC, Comparative cost-effectiveness of SARS-CoV-2 testing strategies in the USA: A modelling study: Lancet Public Health, 2021; 6(3); e184-e91
22. Larremore DB, Toomre D, Parker R, Modeling the effectiveness of olfactory testing to limit SARS-CoV-2 transmission: Nat Commun, 2021; 12(1); 3664
23. Lyng GD, Sheils NE, Kennedy CJ, Identifying optimal COVID-19 testing strategies for schools and businesses: Balancing testing frequency, individual test technology, and cost: PLoS One, 2021; 16(3); e0248783
24. Vander Schaaf NA, Fund AJ, Munnich BV, Routine, cost-effective SARS-CoV-2 surveillance testing using pooled saliva limits viral spread on a residential college campus: Microbiol Spectr, 2021; 9(2); e0108921
25. Drakesmith M, Collins B, Jones A, Cost-effectiveness of a whole-area testing pilot of asymptomatic SARS-CoV-2 infections with lateral flow devices: A modelling and economic analysis study: BMC Health Serv Res, 2022; 22(1); 1190
26. Simonetti M, Zhang N, Harbers L, COVseq is a cost-effective workflow for mass-scale SARS-CoV-2 genomic surveillance: Nat Commun, 2021; 12(1); 3903
27. Wang X, Cai Y, Zhang B, Cost-effectiveness analysis on COVID-19 surveillance strategy of large-scale sports competition: Infect Dis Poverty, 2022; 11(1); 32
28. Marwan M, Winardi W, Mu’ti A, Detection of SARS-CoV-2 Delta variant of concern AY.57 and Clinical characteristics of imported cases on a Vietnamese coal carrier vessel in East Kalimantan, Indonesia: A case report: Journal of Respirology, 2022; 8(2); 99-105
29. Lai CKC, Lam W, Laboratory testing for the diagnosis of COVID-19: Biochem Biophys Res Commun, 2021; 538; 226-30
30. Rao A, Westbrook A, Bassit L, Sensitivity of rapid antigen tests against SARS-CoV-2 Omicron and Delta variants: medRxiv [Preprint], 2023; 2023; 23285583 [Update in: J Clin Microbiol. 2023;2023:e0013823]
31. Dinnes J, Sharma P, Berhane SCochrane COVID-19 Diagnostic Test Accuracy Group, Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection: Cochrane Database Syst Rev, 2022; 7(7); CD013705
32. Panpradist N, Wang Q, Ruth PS, Simpler and faster COVID-19 testing: Strategies to streamline SARS-CoV-2 molecular assays: EBioMedicine, 2021; 64; 103236 [Erratum in: EBioMedicine. 2021;66:103296]
33. Todsen T, Jakobsen KK, Grønlund MP, COVID-19 rapid antigen tests with self-collected vs health care worker-collected nasal and throat swab specimens: A randomized Clinical trial: JAMA Netw Open, 2023; 6(12); e2344295
34. La Marca A, Capuzzo M, Paglia T, Testing for SARS-CoV-2 (COVID-19): A systematic review and clinical guide to molecular and serological in-vitro diagnostic assays: Reprod Biomed Online, 2020; 41(3); 483-99
35. Nguyen HT, Denkinger CM, Brenner S, Cost and cost-effectiveness of four different SARS-CoV-2 active surveillance strategies: Evidence from a randomised control trial in Germany: Eur J Health Econ, 2023; 24(9); 1545-59
36. Guzmán Ruiz Y, Vecino-Ortiz AI, Guzman-Tordecilla N, Cost-effectiveness of the COVID-19 test, trace and isolate program in Colombia: Lancet Reg Health Am, 2022; 6; 100109
37. Neilan AM, Losina E, Bangs AC, Clinical impact, costs, and cost-effectiveness of expanded SARS-CoV-2 testing in Massachusetts: medRxiv [Preprint], 2020; 2020; 20160820 [Update in: Clin Infect Dis. 2021;73(9):e2908–e17
38. Tan SH, Allicock OM, Katamba A, Saliva-based methods for SARS-CoV-2 testing in low- and middle-income countries: Bull World Health Organ, 2022; 100(12); 808-14
39. Pighi L, Henry BM, Mattiuzzi C, Cost-effectiveness analysis of different COVID-19 screening strategies based on rapid or laboratory-based SARS-CoV-2 antigen testing: Clin Chem Lab Med, 2023; 61(9); e168-e171
40. Mahase E, COVID-19: 120 million rapid tests pledged to low and middle income countries: BMJ, 2020; 371; m3857
41. Ansori I, Riefani S, Nurrasyidah I, The correlation of rapid antibody results with sars-CoV-2 PCR in COVID-19 patients in Ulin General Hospital Banjarmasin: Journal of Respirology, 2021; 7(3); 100-5
42. WHO: WHO-COVID-19-global-table-data https://covid19.who.int/table
43. Benoni R, Campagna I, Moretti F, Tardivo S, Comparing swab- and different symptoms-based strategies to ascertain COVID-19 recovery in healthcare workers: A cost-effectiveness analysis: Cost Eff Resour Alloc, 2022; 20(1); 50
44. Maya S, Kahn JG, COVID-19 testing protocols to guide duration of isolation: A cost-effectiveness analysis: BMC Public Health, 2023; 23(1); 864
45. Maya S, McCorvie R, Jacobson K, COVID-19 testing strategies for K-12 schools in California: A cost-effectiveness analysis: Int J Environ Res Public Health, 2022; 19(15); 9371
46. Vilches TN, Rafferty E, Wells CR, Economic evaluation of COVID-19 rapid antigen screening programs in the workplace: BMC Med, 2022; 20(1); 452
Tables
 Table 1. Characteristics of selected studies.
Table 1. Characteristics of selected studies. Table 2. Economic evaluation, cost outcome, and health related quality of life.
Table 2. Economic evaluation, cost outcome, and health related quality of life. Table 3. Model input for cost analysis of COVID-19 testing in high-income countries (HIC), upper-middle-income countries (UMIC), lower-middle-income countries (LMIC), and low-income countries (LIC) for the post-pandemic era.
Table 3. Model input for cost analysis of COVID-19 testing in high-income countries (HIC), upper-middle-income countries (UMIC), lower-middle-income countries (LMIC), and low-income countries (LIC) for the post-pandemic era. Table 1. Characteristics of selected studies.
Table 1. Characteristics of selected studies. Table 2. Economic evaluation, cost outcome, and health related quality of life.
Table 2. Economic evaluation, cost outcome, and health related quality of life. Table 3. Model input for cost analysis of COVID-19 testing in high-income countries (HIC), upper-middle-income countries (UMIC), lower-middle-income countries (LMIC), and low-income countries (LIC) for the post-pandemic era.
Table 3. Model input for cost analysis of COVID-19 testing in high-income countries (HIC), upper-middle-income countries (UMIC), lower-middle-income countries (LMIC), and low-income countries (LIC) for the post-pandemic era. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952