27 April 2024: Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourth-Year PharmD Undergraduates from Riyadh, Saudi Arabia
Wajid Syed1ABCDEFG*, Mahmood Basil A. Al-Rawi2ABCDEFG, Adel Bashatah3ACEGDOI: 10.12659/MSM.943468
Med Sci Monit 2024; 30:e943468
Abstract
BACKGROUND: Clinical Trials (CTs) are essential for the formation of a new medicine. This questionnaire-based study included 179 male third- and fourth-year PharmD undergraduate students from Riyadh, Saudi Arabia, between 18 and 23 years of age, was conducted in 2022, and aimed to evaluate student knowledge and attitudes toward CTs.
MATERIAL AND METHODS: A cross-sectional study was conducted using self-administered questionnaires. The data was collected using convenience sampling. Part 1 of the study collected demographics, with 6 items; part 2 measured knowledge about CTs (9 items); part 3 measured attitudes (5 items) measured on a 5-point Likert scale. A score of 1 was given for correct, and a score of 0 for each incorrect response. Multiple linear regression was used to evaluate the determinants of knowledge and attitudes about CTs.
RESULTS: A total of 108 (60.3%) undergraduates reported having heard about CTs, 15% of undergraduates had heard about institutional review boards, while 74.3% of the undergraduates believed that CTs have direct benefits to society. The mean knowledge score of the CTs among the students was 5.75±2.32 (median 6.00), while the mean attitude score of CTs was 16.45±4.56 (median 16.00). However, the results of the simple regression model revealed that age, year of study, and chronic disease status were not predictors of the knowledge and attitude score (P>0.05).
CONCLUSIONS: This study indicated that half of undergraduates were aware of CTs, were knowledgeable, and showed positive attitudes. Furthermore, the study identified potential misunderstandings about the benefits of CTs.
Keywords: Biomarkers, Pharmacological, Clinical Trials as Topic, Health Knowledge, Attitudes, Practice, Knowledge
Introduction
Clinical trials (CTs) are medical studies in which participants volunteer to participate and are widely used to study the effects of medications and medical treatments [1–3]. Modern medicine relies heavily on CTs, one of the fastest and safest ways to find new treatments for patients. In addition, CTs show results in treatment efficacy and safety and help in the global development of the healthcare system [1–3]. However, enrolling and recruiting patients for CTs is presently one of the most significant challenges faced by researchers, nationally and internationally [2,3]. The most cited reasons were reported to be fear of adverse effects of new drugs and inadequate awareness about CTs [3,4].
Human participants are only now becoming aware of the concept of participating in CTs in developed countries, although this concept is largely unknown in less developed countries [3,4]. The study of human participants in CTs aims to answer specific questions, including the identification of new treatments and well-known interventions that merit further research and comparison [3,4]. To ensure the safety and success of CTs, ethical approval is required, and the approval is granted by the health authorities and ethics committees in the country or organization in which the research is conducted [3,4]. Approval from these authorities does not imply that the therapy is “safe” or “effective”, but it does mean that the trial can be conducted [3,4]. Successful CTs depend greatly on the knowledge and attitude of patients and individuals toward CTs.
Studies have highlighted the knowledge of and attitude toward CTs among the public in Saudi Arabia, Jordan, and Denmark [5–7]. For instance, an online study found that approximately 79.9% of studied participants knew about CTs, and at least 9.8% had previously participated in CTs [8]. On the other hand, another study revealed that only 21.8% of individuals correctly recognized what a CT was, and only 1.2% had participated in CTs. Research in Saudi Arabia shows that 85.9% of participants stated that CTs increased their medical expertise. Nonetheless, 20 (10%) of earlier participants believed CTs had no benefits, while 9.1% were unfamiliar with CTs, and 73.8% supported the CTs program [5]. Furthermore, the literature in Saudi Arabia revealed that 64.6% of Saudi adults had never heard of CTs. More than half of the participants had poor knowledge of and attitudes towards CTs [9]. According to the US Food and Drug Administration (US FDA), the Middle East and North Africa region contributes to less than 1% of global CTs [10], However, the Middle East North America region ranks only around 2% of the countries involved in CTs enrolled by pharmaceutical companies, as per a 2015–2016 CTs report released by the US FDA [10].
In the last few decades, research and development, especially clinical research in Saudi Arabia, has drastically advanced; but still, the numbers of CTs in the Kingdom are insufficient [11,12]. Under Vision 2030, national regulatory bodies in Saudi Arabia, such as the Saudi Food and Drug Authority (SFDA), implemented CT administration [11,12]. This administration provides services such as regulation, registration, implementing guidelines, ensuring the safety of volunteers, increasing the awareness of the public as well as researchers, and creating a database, like the Saudi CTs Registry, to record all CTs conducted in the Kingdom of Saudi Arabia [11,12]. Thus, further studies would be helpful to clarify individuals’ interests and concerns regarding participating in CTs. A successful CT relies heavily on the recruitment of individuals, utilizing volunteers or non-volunteers [11,12].
The recruitment of a sufficient number of participants is critical in completing the objectives of the CTs, which are to test the research hypothesis and answer the research questions [13,14]. Failure to attract a suitable number of participants can result in a waste of time, money, and effort [13,14]. Therefore, volunteers’ knowledge, attitudes, and awareness about CTs are challenging aspects in achieving the required objectives through recruitment of study volunteers [15,16]. There is evidence that increased knowledge promotes a positive attitude toward CTs, which can promote higher rates of participation [15,16]. Studies have examined the knowledge and attitudes of CTs among the public in Saudi Arabia and other countries [17,18], yet studies among healthcare students in other countries reported poor knowledge, awareness, and attitudes of CTs [19,20]. Furthermore, there is insufficient research regarding the knowledge and attitudes of CTs in the setting of Saudi healthcare students, and international studies concerning undergraduates in this context are very limited.
The role of pharmacists in CTs is widespread [21,22]. For instance, pharmacists can improve patient involvement in CTs by communicating the significance of CTs with the patients [21,22]. Additionally, pharmacists can oversee the administration of investigational items, which can include biologicals and medications, as well as gene therapy and radiopharmaceuticals [21,22]. Therefore, having adequate knowledge, positive attitudes, and awareness of various aspects of CTs is crucial for pharmacists. Moreover, pharmacists are often the first medical professionals that people turn to for advice. In this view of pharmacy students, adequate knowledge at their graduation not only helps in achieving excellent grades but also helps at their practice site as pharmacists. As per the literature search, no such research has been conducted so far among Saudi undergraduate pharmacy students to examine the knowledge of and attitudes toward CTs. Therefore, this questionnaire-based study, including 179 male third- and fourth-year PharmD undergraduates from Riyadh, Saudi Arabia, between 18 and 23 years of age, was conducted over 3 months in 2022 and aimed to evaluate the students’ knowledge of and attitudes toward CTs.
Material and Methods
STUDY DESIGN, SETTING, AND POPULATION:
Prior to data collection, the King Saud University Institutional Review Board (IRB; reference number KSU-HE-24-033) in Riyadh, Saudi Arabia, approved the study protocol and questionnaires. Additionally, the students gave their informed consent after being told that the data would be used only for research purposes and that confidentiality during the study would be upheld. Furthermore, students were informed that they could with withdraw from the study at any time. A cross-sectional study was conducted among pharmacy undergraduates to assess their knowledge, awareness, and attitudes of CTs. It was a prospective self-reporting survey using online questionnaires conducted from August to October 2022. The inclusion criteria were as follows: undergraduate student aged >18 years, male sex, able to speak both Arabic and English, currently pursuing courses at the study site, belonging to third and fourth years of PharmD courses at Saudi university in Riyadh, and willing to complete the survey after signing the informed consent. Those who did not match the inclusion criteria and female students were excluded from the study.
SAMPLE SIZE ESTIMATION:
The required sample size was calculated similarly to previous studies [23–25] using an electronic calculator, namely Raosoft, with a 95% confidence level and pre-determined margin of error of 5%. During the study period, we identified approximately 300 students pursuing their courses on campus. Because we were unsure of the potential outcomes for each question, we assumed that the response distribution for each question would be 50%. The calculated sample size was 169, but we approached 250 students to avoid sample bias.
QUESTIONNAIRE DESIGN:
A semi-structured questionnaire was adopted from previous studies to evaluate the undergraduates’ knowledge and attitudes of CTs [5,8–10]. The survey questionnaire was designed in Arabic with the help of a native Arabic speaker; later the questionnaires were subjected to forward and backward translation into the English language. The questionnaires were divided into 3 parts: the first part collected demographic characteristics, such as age, presence of chronic disease, and insurance status (6 items). The second part of the study included knowledge- and awareness-related questions about CTs, for instance, the definition of a clinical trial, ethical regulations, and direct or indirect benefits of CTs (9 items). All the knowledge and awareness questions were assessed using a multiple-choice format. The third and last section of the study measured the attitudes of undergraduates toward CTs (5 items). The participants rated their attitudes using a Likert-type tool, ranging from agreeing to strongly disagreeing with each item.
Two stages went into the validation of the questionnaire. First, a research expert in the relevant field assessed the draft of the questions to ensure that the questionnaire’s content and flow were accurate. Second, a pilot study was conducted with a randomly chosen sample of 30 students to gather feedback, determine the feasibility of the study, and pre-test the questionnaire. Cronbach’s alpha was computed as a reliability test, and the results showed that the knowledge and awareness questions had a Cronbach’s alpha of 0.79, while the attitude questions had 0.85, suggesting that the questionnaire was valid and reliable to conduct the study.
DATA COLLECTION PROCEDURE:
The data were collected using convenience-sampling methods, using online survey tools created with Google Forms. The data collection was conducted using social media platforms, such as WhatsApp. Emails and other techniques were considered, to increase the response rate. The participants received an invitation link with a survey form attached. The knowledge score for the CTs was computed using knowledge items (n=10). A score of 1 was given for a correct answer and a score of 0 for an incorrect answer. The overall score was calculated using sum of the total items. The knowledge score was divided into 2 categories: good (scoring more than 50% of the overall score) and poor (scoring less than 50% of the total score). Similarly, the rating scale (strongly disagree=1; disagree=2; neutral=3; strongly agree=4; agree=5) was computed to create the attitude scores. The attitude score was further divided into 2 categories: good (scoring more than 50% of the overall score) and poor (scoring less than 50% of the total score).
STATISTICAL ANALYSIS:
The Statistical Package for Social Sciences version 27.0 (IBM Corp, Armonk, NY, USA) was used to evaluate the data; for categorical variables, data are presented as percentages (%) and frequencies (n). To find the association between the knowledge levels and demographics of the students, the chi-Square or Fisher exact test was used. To identify potential predictors of students’ characteristics and their knowledge and attitudes of CTs, a multiple linear regression analysis was conducted, with a
Results
SOCIO-DEMOGRAPHIC CHARACTERISTICS:
A total of 179 undergraduates responded to the questionnaires, giving a response rate of 71.6% (total n=250). All students were male, most aged between 18 and 23 years (72.6%), 95% of were Saudi nationals (n=170), 126 (70.4%) were in the fourth year of the PharmD program, 63.1% had no health insurance, and 12.8% had a history of chronic disease. The baseline characters of the undergraduates are shown in Table 1.
KNOWLEDGE AND AWARENESS OF UNDERGRADUATES TOWARD CTS:
Regarding the students’ knowledge about CTs, a total of 108 (60.3%) undergraduates reported having heard about CTs. Studies to test new drugs or procedures in humans were the most common definition (58.2%) of CTs reported by undergraduates. Only 15% of the undergraduates had heard about IRBs (Figure 1). The FDA was most commonly recognized by undergraduates (94.4%).
More than half of the undergraduates (55.9%) reported that the SFDA plays a role in organizing CTs, 136 undergraduates were aware that there are ethical guidelines for regulating CTs. More than one-third of the undergraduates were sure that there are direct benefits for clinical trial participants, whereas 74.3% of the undergraduates believed that CTs have direct benefits to society. Most undergraduates (53.1%) were aware of the conditions for initiating CTs, 14% of the undergraduates reported that the investigator could start the CTs only with the consent of the participants/patients, while 23.5% of them were not sure about it. Figure 2 shows the students; knowledge of how to begin the CTs by investigators. More than half of the undergraduates (52%) knew that CT participants could withdraw from a clinical study at any time. The knowledge-related responses are given in Table 2.
ATTITUDE OF UNDERGRADUATES STUDENTS TOWARD CTS:
Concerning the attitude towards CTs, three-quarters of the undergraduates (38.5%) gave positive responses about testing new drugs on adults, while most (67.1%) gave positive responses about testing approved drugs. Nevertheless, only 43.5% of them were in favor of conducting CTs on healthy volunteers. In contrast, only 16.8% of those surveyed agreed with the testing of new drugs on pediatric patients, whereas 40.8% agreed with testing approved/off-label drugs on pediatric patients. A total of 14.5% were willing to consider participating in a CT if they or a close relative were offered the opportunity, and 81% stated that drug development depends heavily on understanding CTs. Detailed information on the attitude toward CTs is illustrated in Table 3. The overall mean attitude score was 16.45±4.565 (median,16.00; range, 0–19).
KNOWLEDGE AND ATTITUDE LEVELS OF CTS:
In this study, 105 (59%) students had a good knowledge score and 101 (56.4%) had positive attitudes, while 73 (41%) of students were found to have poor knowledge of CTs, and 78 (43.6%) reported unfavorable attitudes towards CTs, as shown in Figure 3.
ASSOCIATION BETWEEN KNOWLEDGE AND ATTITUDES OF CTS AND DEMOGRAPHIC CHARACTERS:
The knowledge and attitudes scores were not significantly associated with age (P=0.371), course of study (P=0.093), nationality (P=0.0957), marital status (P=0.094), and chronic disease status (P=0.992). Further, a detailed explanation of the association between knowledge and attitudes levels and demographics of the participants is shown in Table 4. However, the results of the multiple linear regression model revealed no significant association between attitude score, age, year of study, nationality, marital status, and presence of chronic disease, and these variables were not predictors of the knowledge (P>0.05) and attitude scores (P>0.05), as shown in Tables 5 and 6.
Discussion
LIMITATIONS:
The present study had a few limitations. First, the results were derived from a single university in one region of Saudi Arabia and, therefore, the findings cannot be generalizable to other regions in Saudi Arabia and other countries. Second, the questionnaire was self-administered, which would have increased the likelihood of biases, such as response bias or social desirability bias. Third, the study focused on male students and excluded female students because it was found that male students had better access to the questionnaire, since in Saudi Arabia, men and women have different campuses and men are not allowed to enter the women’s campus, due to the country’s culture. Therefore, we support the introduction of courses or programs that inform students about the misconceptions about CTs and their implications for general health. An interprofessional approach is required for the educational system. Furthermore, the most practical method to handle such a difficult issue is by providing additional education on CTs to pharmacists, other professionals, and patients regarding the relevance of CTs in the discoveries in healthcare and the safety of patients and participants in CTs.
Conclusions
This study highlights that half of undergraduates were aware of CTs and had good knowledge and positive attitudes. Despite having acceptable knowledge, many undergraduates were not aware of the existence of IRBs. Additionally, the study revealed the existence of potential misunderstandings about the benefits of CT participation, which can foster a negative attitude toward CTs and limit CT sample sizes. More importantly, the number of individuals participating in CTs is declining, both in national and international studies [30,31], which is associated with negative consequences on the development of new drugs or treatments. Finally, we recommend that healthcare professionals and the collective engagement through social media can help improve awareness, knowledge, and attitudes toward CTs, thereby correcting misconceptions and improving the rates of participation in clinical trials.
Figures
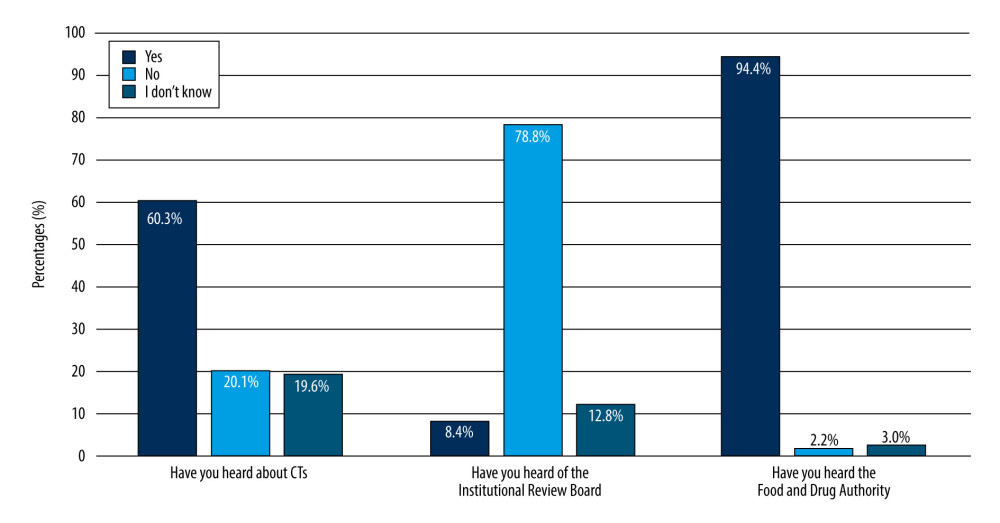 Figure 1. Awareness of 179 male third- and fourth-year PharmD students of clinical trials.
Figure 1. Awareness of 179 male third- and fourth-year PharmD students of clinical trials. 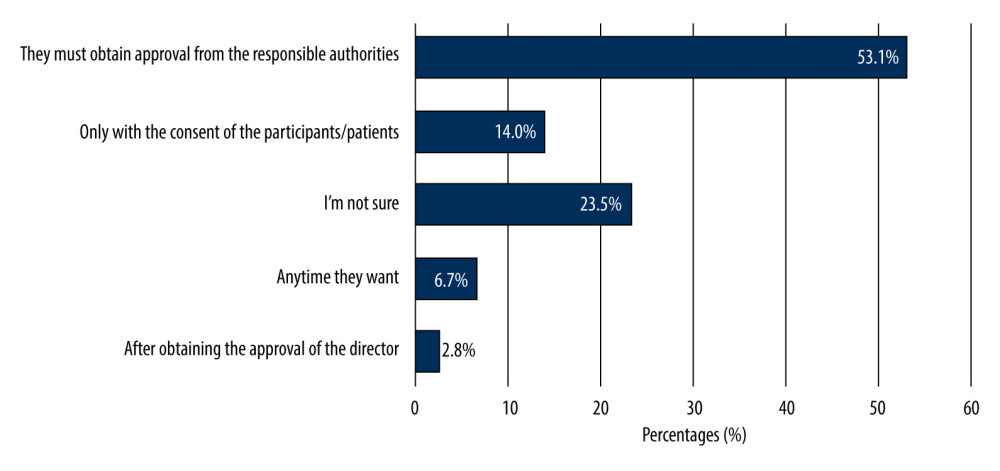 Figure 2. Knowledge of 179 male third- and fourth-year PharmD students about when to begin clinical trials.
Figure 2. Knowledge of 179 male third- and fourth-year PharmD students about when to begin clinical trials. 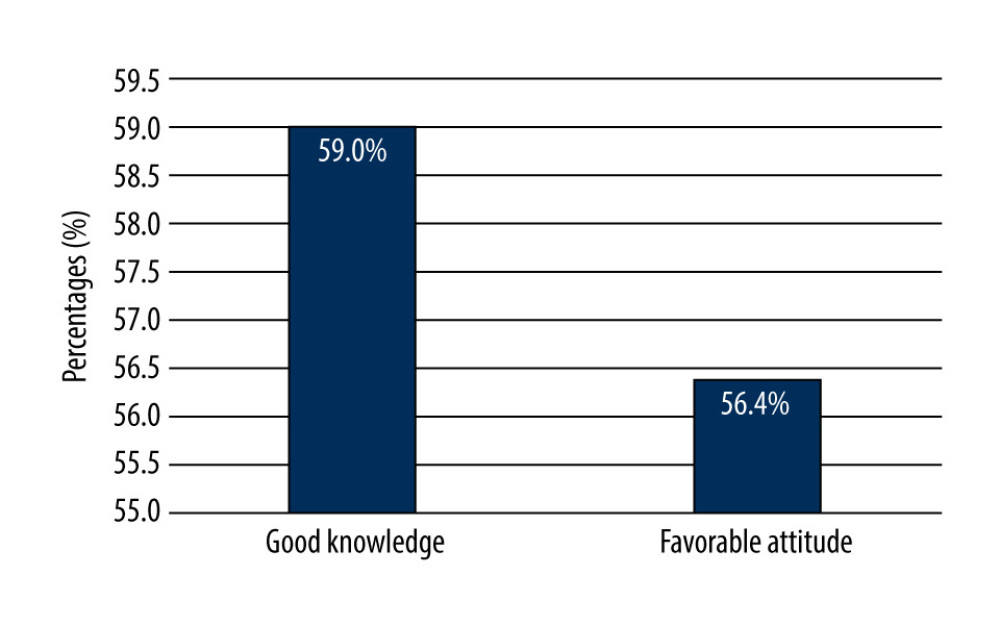 Figure 3. Knowledge and attitudes levels of clinical trials among 179 male third- and fourth-year PharmD students.
Figure 3. Knowledge and attitudes levels of clinical trials among 179 male third- and fourth-year PharmD students. Tables
Table 1. Demographic characters of the 179 male third- and fourth-year PharmD students.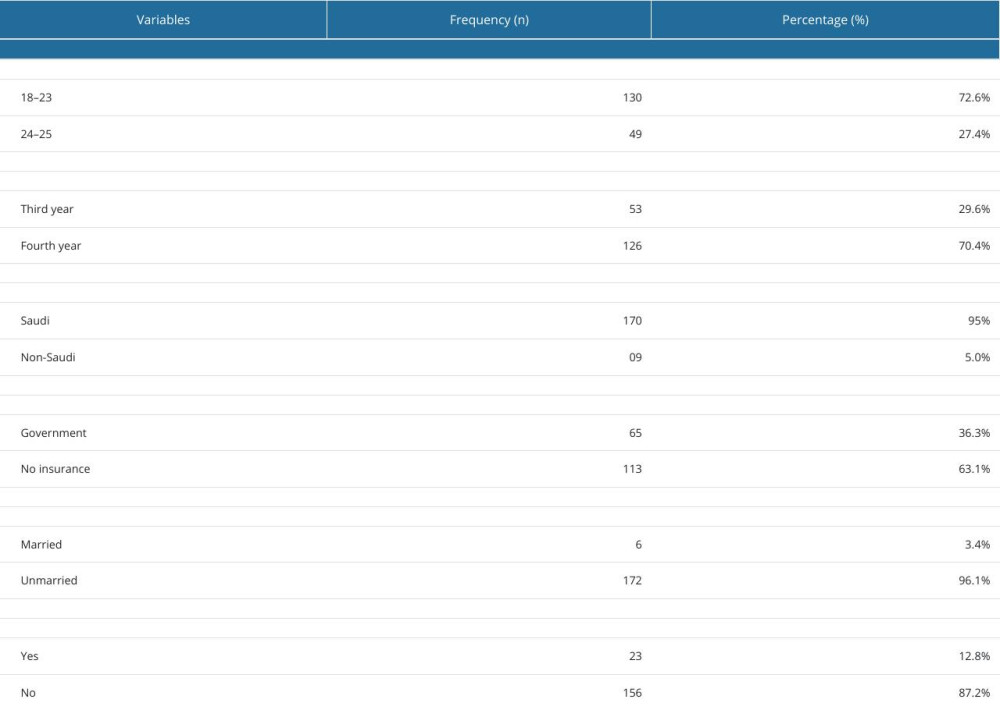 Table 2. Students’ frequency of responses to questions regarding clinical trials.
Table 2. Students’ frequency of responses to questions regarding clinical trials.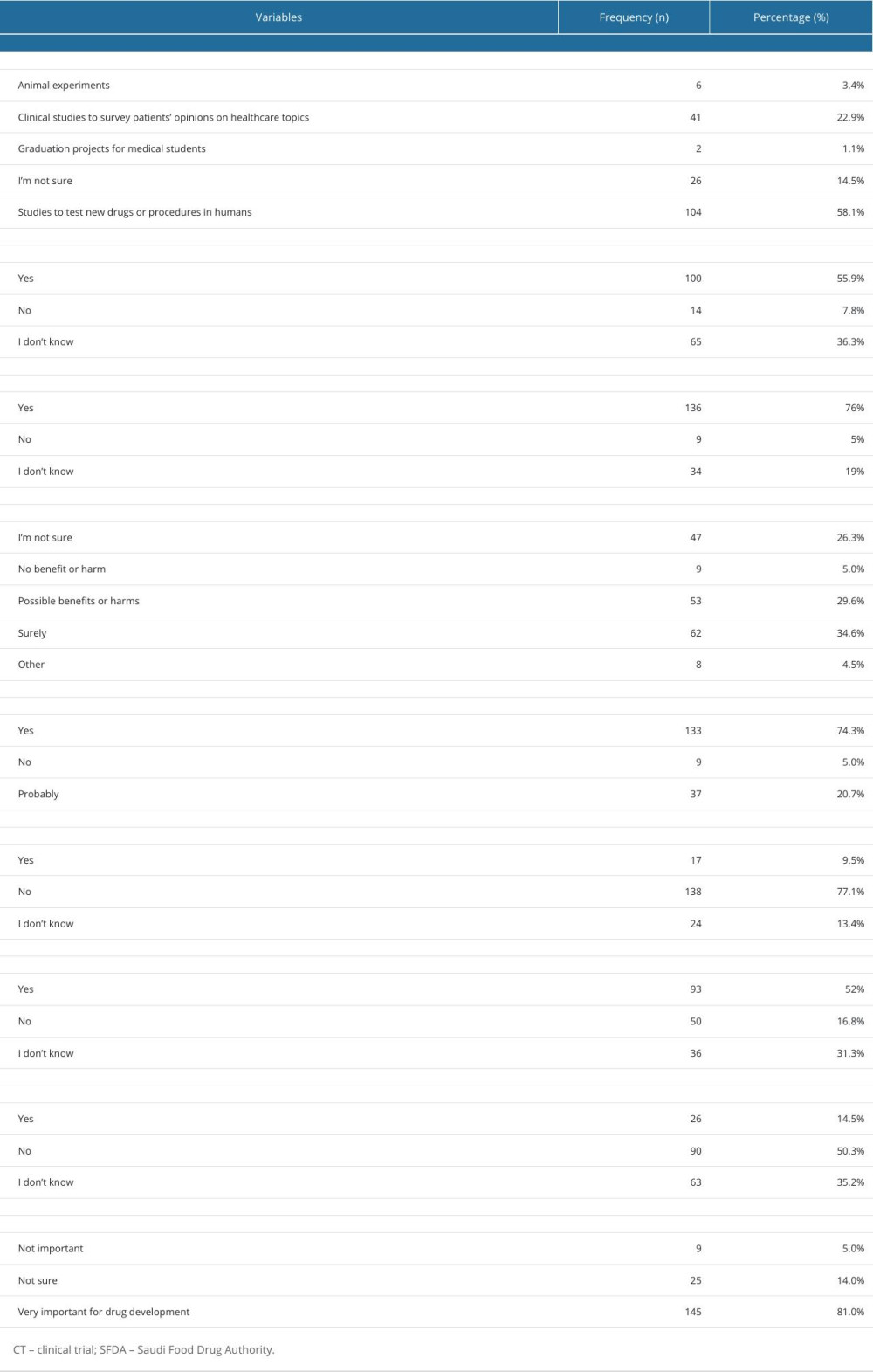 Table 3. Attitudes of 179 Male Third- and Fourth-Year PharmD students toward clinical trials.
Table 3. Attitudes of 179 Male Third- and Fourth-Year PharmD students toward clinical trials.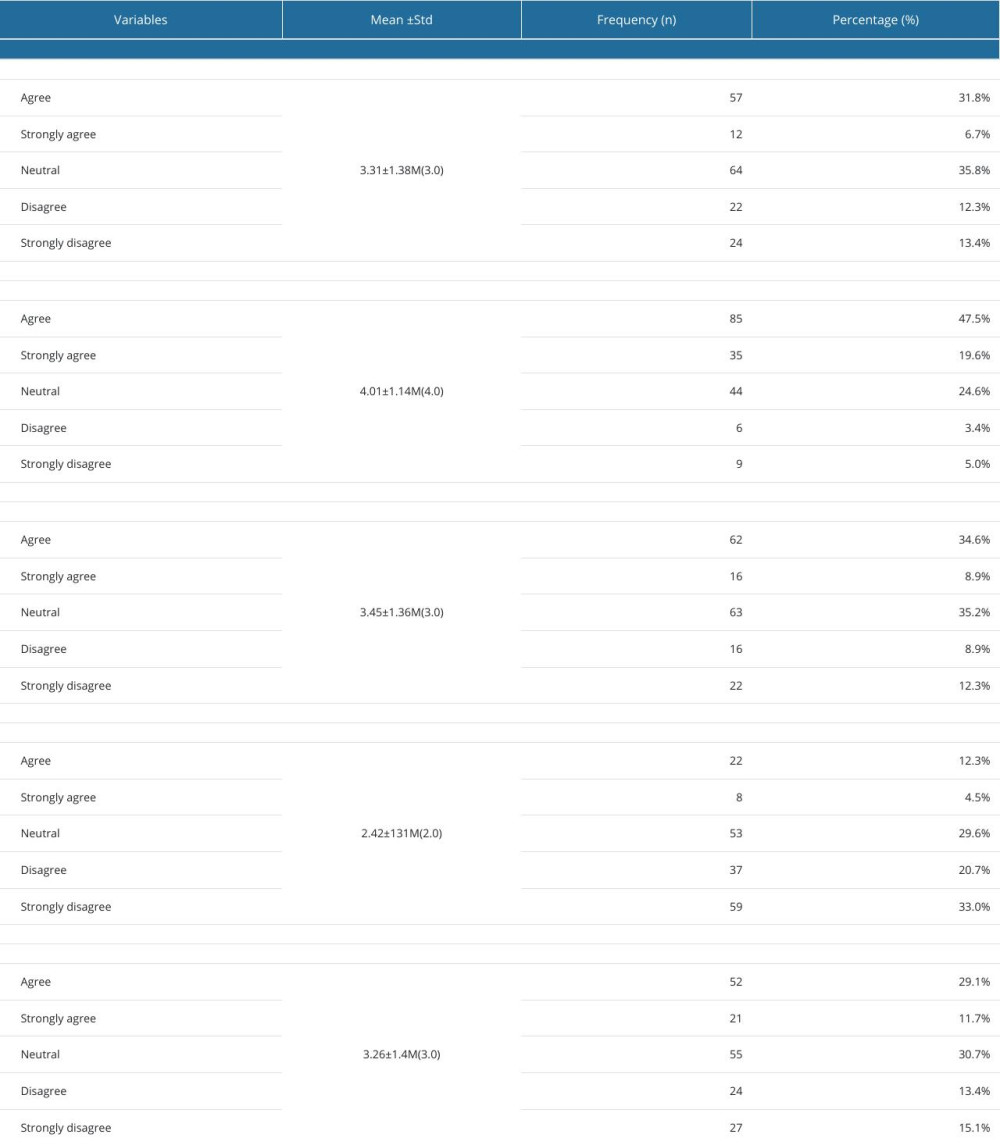 Table 4. Comparison of knowledge and attitudes scores of 179 male third- and fourth-year PharmD students according to socio-demographic variables.
Table 4. Comparison of knowledge and attitudes scores of 179 male third- and fourth-year PharmD students according to socio-demographic variables.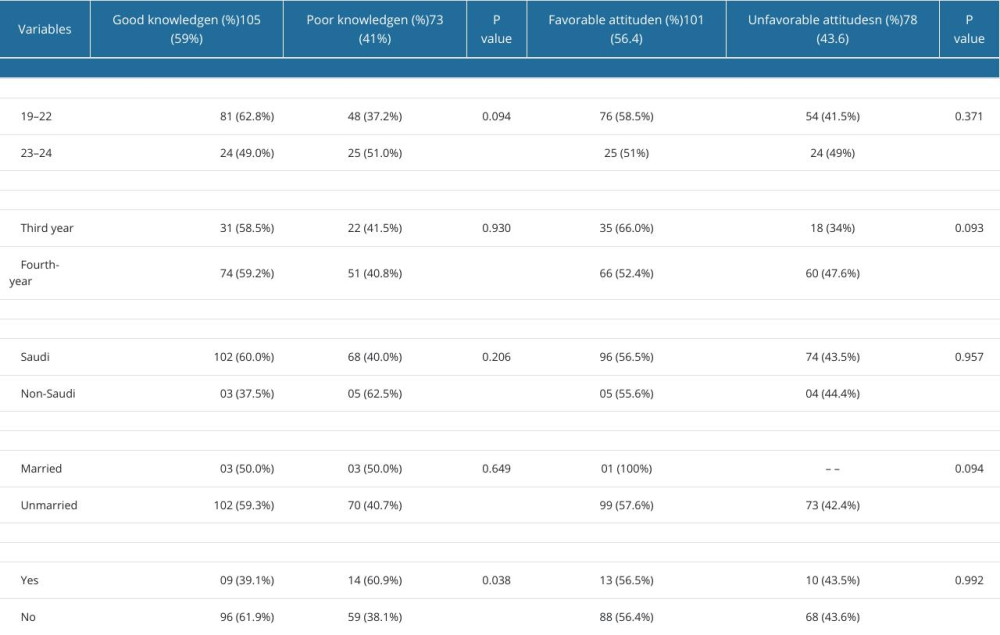 Table 5. Multiple linear regression model for predictors of attitude score.
Table 5. Multiple linear regression model for predictors of attitude score.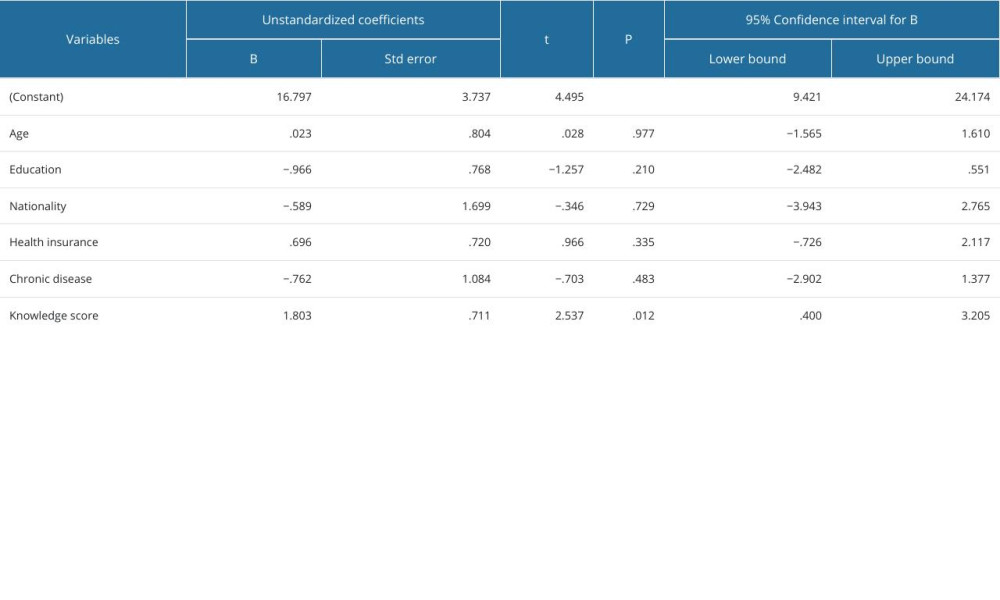 Table 6. Multiple linear regression model for predictors of knowledge score.
Table 6. Multiple linear regression model for predictors of knowledge score.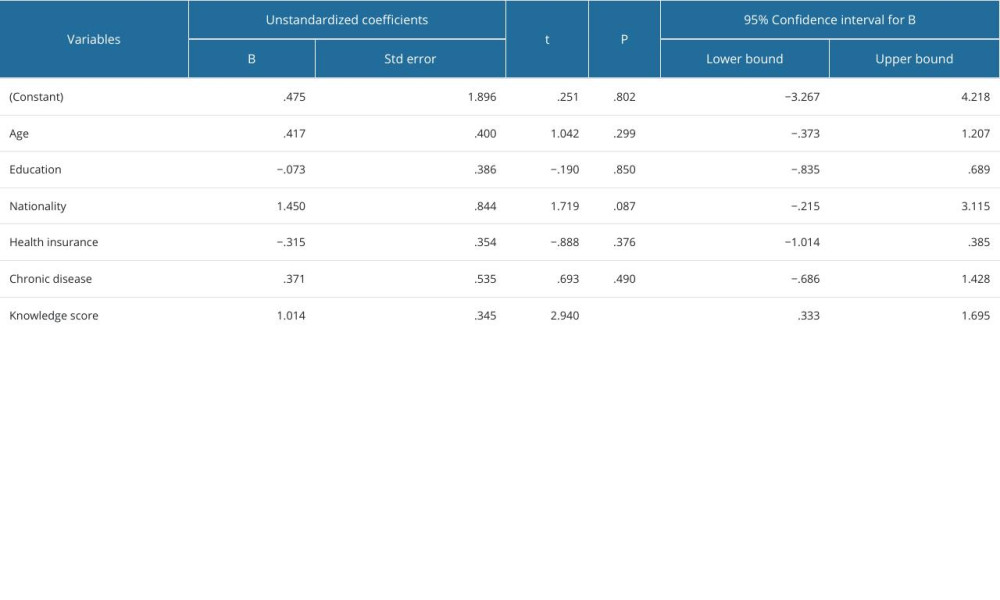
References
1. Roache: What is a clinical trial and how does a trial work? Available from: https://www.roche.com/innovation/process/clinical-trials/about#2a13f587-0452-4bf7-8da9-1c2d6bba4e6c
2. Oxford Centre for Evidence-Based Medicine (OCEBM), 2011 Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
3. US National Institutes of Health, Learn about clinical studies – ClinicalTrials.gov [Internet]: ClinicalTrials.gov, 2012 Available from: https://clinicaltrials.gov/ct2/about-studies/learn
4. Toerien M, Brookes ST, Metcalfe C, A review of reporting of participant recruitment and retention in RCTs in six major journals: Trials, 2009; 10; 52
5. Al-Tannir MA, El-Bakri N, Abu-Shaheen AK, Knowledge, attitudes, and perceptions of saudis towards participating in clinical trials: PLoS One, 2016; 11(2); e0143893
6. Ahram M, Farkouh A, Haddad M, Knowledge of, attitudes to and participation in clinical trials in Jordan: A population-based survey: East Mediterr Health J, 2020; 26(5); 539-46
7. Madsen SM, Mirza MR, Holm S, Attitudes towards clinical research amongst participants and nonparticipants: J Intern Med, 2002; 251(2); 156-68
8. Ohmann C, Deimling A, Attitude towards clinical trials: Results of a survey of persons interested in research: Inflamm Res, 2004; 53(Suppl 2); S142-S47
9. Abouelkheir M, Taha AE, Thirunavukkarasu A, Knowledge and attitude towards clinical trials among general population of Northern Saudi Arabia during COVID-19 era: A cross-sectional study: Healthcare (Basel), 2023; 11(5); 680
10. Nair SC, Ibrahim H, Celentano DD, Clinical trials in the Middle East and North Africa (MENA) Region: Grandstanding or grandeur?: Contemp Clin Trials, 2013; 36(2); 704-10
11. Innovations: King Abdulla international medical research center (KAIMRC). The importance of clinical trials for Saudi healthcare Available From: https://innovations.kaimrc.med.sa/en/editorial/421/the-importance-of-clinical-trials-for-saudi-healthcare
12. Saudi Gazette: Leading the way. Value of conducting clinical trials in Saudi Arabia rising Available from: https://saudigazette.com.sa/article/519373
13. Fogel DB, Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review: Contemp Clin Trials Commun, 2018; 11; 156-64
14. Briel M, Elger BS, McLennan S, Schandelmaier S, Exploring reasons for recruitment failure in clinical trials: A qualitative study with clinical trial stakeholders in Switzerland, Germany, and Canada: Trials, 2021; 22(1); 844
15. Abu Farha R, Alzoubi KH, Khabour OF, Mukattash TL, Factors influencing public knowledge and willingness to participate in biomedical research in Jordan: A national survey: Patient Prefer Adherence, 2020; 14; 1373-79
16. Awwad O, Maaiah S, Almomani BA, Clinical trials: Predictors of knowledge and attitudes towards participation: Int J Clin Pract, 2021; 75(3); e13687
17. Al-Rawashdeh N, Damsees R, Al Al-Jeraisy M, Knowledge of and attitudes toward clinical trials in Saudi Arabia: A cross-sectional study: BMJ Open, 2019; 9(10); e031305
18. Al-Tannir MA, El-Bakri N, Abu-Shaheen AK, Knowledge, attitudes and perceptions of Saudis towards participating in clinical trials: PLoS One, 2016; 11(2); e0143893
19. Vittalrao AM, Kumari KS, Gill R, Thomson SR, A questionnaire survey on awareness of clinical trials among medical students: Biomed Pharmacol J, 2018; 11(4); 2005-9
20. Sharma KH, Jindal A, Low awareness of clinical research in India amongst finnal year medical students and physicians: Need for increased emphasis on clinical research in medical curriculum: Arch Med Health Sci, 2014; 2; 234-37
21. Ali S, Karakitsos D, Clinical pharmacists as principal investigators in clinical trials: Encyclopedia of Pharmacy Practice and Clinical Pharmacy Elsevier, 2019; 81-93
22. Myoclinic College of Medicine and Science: Behind The Scenes, Pharmacists Play Key Role in Clinical Research Available from: https://college.mayo.edu/about/news/news-archive/behind-the-scenes-pharmacists-play-key-role-in-clinical-research/
23. Syed Snr W, Bashatah A, Al-Rawi AMB, Evaluation of knowledge of food-drug and alcohol-drug interactions among undergraduate students at King Saud University – an observational study: J Multidiscip Healthc, 2022; 15; 2623-33
24. Bashatah AS, Syed W, Al-Rawi MB, Al Arifi MN, Assessment of headache characteristics, impact, and managing techniques among pharmacy and nursing undergraduates – an observational study: Medicina, 2023; 59(1); 130
25. Samreen S, Siddiqui NA, Mothana RA, Prevalence of anxiety and associated factors among pharmacy students in Saudi Arabia: A cross-sectional study: Biomed Res Int, 2020; 2020; 2436538
26. Al-Lawati H, Al-Baimani K, Al-Zadjali M, Knowledge and attitudes towards clinical trial participation in Oman: A cross-sectional study: Sultan Qaboos Univ Med J, 2018; 18(1); e54-e60
27. Chatterjee B, Sarkar J, Awareness of medical ethics among undergraduates in a West Bengal medical college: Indian J Med Ethics, 2012; 9(2); 93-100
28. Sheblaq NR, Traifi S, Sakiani MA, Awareness and attitude of cancer patients about participation in clinical research (CR) in Saudi Arabia: J Clin Oncol, 2013; 15; e17528
29. Akhondzadeh S, Mostafavi SA, Keshavarz SA: J Clin Pharm Ther, 2020; 45(1); 134-43
30. Sedrak MS, Ji J, Tiwari A, Clinical trial enrollment, ineligibility, and reasons for decline in older vs younger patients with cancer in the National Cancer Institute community oncology research program: JAMA Network Open, 2022; 5(10); e2235714
31. Strayer TE, Hollingsworth EK, Shah AS, Why do older adults decline participation in research? Results from two deprescribing clinical trials: Trials, 2023; 24(1); 456
Figures
 Figure 1. Awareness of 179 male third- and fourth-year PharmD students of clinical trials.
Figure 1. Awareness of 179 male third- and fourth-year PharmD students of clinical trials. Figure 2. Knowledge of 179 male third- and fourth-year PharmD students about when to begin clinical trials.
Figure 2. Knowledge of 179 male third- and fourth-year PharmD students about when to begin clinical trials. Figure 3. Knowledge and attitudes levels of clinical trials among 179 male third- and fourth-year PharmD students.
Figure 3. Knowledge and attitudes levels of clinical trials among 179 male third- and fourth-year PharmD students. Tables
 Table 1. Demographic characters of the 179 male third- and fourth-year PharmD students.
Table 1. Demographic characters of the 179 male third- and fourth-year PharmD students. Table 2. Students’ frequency of responses to questions regarding clinical trials.
Table 2. Students’ frequency of responses to questions regarding clinical trials. Table 3. Attitudes of 179 Male Third- and Fourth-Year PharmD students toward clinical trials.
Table 3. Attitudes of 179 Male Third- and Fourth-Year PharmD students toward clinical trials. Table 4. Comparison of knowledge and attitudes scores of 179 male third- and fourth-year PharmD students according to socio-demographic variables.
Table 4. Comparison of knowledge and attitudes scores of 179 male third- and fourth-year PharmD students according to socio-demographic variables. Table 5. Multiple linear regression model for predictors of attitude score.
Table 5. Multiple linear regression model for predictors of attitude score. Table 6. Multiple linear regression model for predictors of knowledge score.
Table 6. Multiple linear regression model for predictors of knowledge score. Table 1. Demographic characters of the 179 male third- and fourth-year PharmD students.
Table 1. Demographic characters of the 179 male third- and fourth-year PharmD students. Table 2. Students’ frequency of responses to questions regarding clinical trials.
Table 2. Students’ frequency of responses to questions regarding clinical trials. Table 3. Attitudes of 179 Male Third- and Fourth-Year PharmD students toward clinical trials.
Table 3. Attitudes of 179 Male Third- and Fourth-Year PharmD students toward clinical trials. Table 4. Comparison of knowledge and attitudes scores of 179 male third- and fourth-year PharmD students according to socio-demographic variables.
Table 4. Comparison of knowledge and attitudes scores of 179 male third- and fourth-year PharmD students according to socio-demographic variables. Table 5. Multiple linear regression model for predictors of attitude score.
Table 5. Multiple linear regression model for predictors of attitude score. Table 6. Multiple linear regression model for predictors of knowledge score.
Table 6. Multiple linear regression model for predictors of knowledge score. In Press
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








