28 March 2023: Database Analysis
A Novel Immune-Related Signature to Predict Prognosis and Immune Infiltration of Cervical Cancer
Yun Lin1ABCDE, Rendong Zhang2BCD, Hongchao Pan1CDG, Yaochen Li1AEG*DOI: 10.12659/MSM.938660
Med Sci Monit 2023; 29:e938660
Abstract
BACKGROUND: Cervical cancer is one of the most common malignances among women globally. This study aimed to construct a novel immune-related signature to predict the prognosis and immune infiltration of cervical cancer.
MATERIAL AND METHODS: Transcriptomic profiles and corresponding clinical information of cervical cancer patients were obtained from The Cancer Genome Atlas (TCGA) database and GEO database. The hub immune-related genes were screened and selected using Cox regression analysis and LASSO regression analysis. A novel signature was established based on the expression levels and corresponding coefficients of the selected hub immune-related genes. Kaplan-Meier survival curve and ROC curve illustrated the prognostic value of this novel signature in cervical cancer. The predictive accuracy and stability of this novel signature were confirmed in the validation cohort, internal testing set and external testing set. Then, a nomogram was constructed to predict individual survival probability of cervical cancer patient. The association between the risk scores of novel signature and immune infiltration was investigated through single-sample gene set enrichment analysis (ssGSEA).
RESULTS: Ten hub immune-related genes (TFRC, SPP1, CAMP, CSF2, TUBB3, ZAP70, CHIT1, LEPR, DLL4, and DES) were selected to construct a novel signature. The risk score of this novel signature could be an independent prognostic factor in cervical cancer, which divided patients into high-risk and low-risk groups. The patients in high-risk groups showed significantly worse overall survival rates than those in low-risk groups in all training and validation cohorts (all P<0.05). A nomogram model was constructed based on the risk score of the novel signature and other clinical characteristics, which achieved the highest clinical net benefit across the entire range of reasonable threshold probabilities (concordance index=0.813). Furthermore, gene enrichment analysis revealed that the novel signature was closely related with immunology. The novel signature was negatively correlated with the infiltration of most immune cell types, especially T cell subsets (P<0.001).
CONCLUSIONS: The novel signature could comprehensively predict the prognosis and immune infiltration of cervical cancer. It may provide new insights for the precise treatment in cervical cancer.
Keywords: Immune Checkpoint Proteins, Risk Factors, Transcriptome, Humans, Female, Prognosis, nomograms, Databases, Factual
Background
Cervical cancer is the fourth most common cancer and the leading cause of cancer-related death among women globally [1]. Ascribed to improvement of socio-economic levels and diminishing prevalence of sexually transmitted disease, the incidence rate of cervical cancer has decreased in many countries for the past few decades [2,3]. However, the mortality rate remains high and the median survival time of advanced cervical cancer patients is only 16.8 months [4,5]. According to the estimates produced by International Agency for Research on Cancer, there were 342 000 deaths caused by cervical cancer in 2020 worldwide [1]. Consequently, novel and effective therapeutic strategies are urgently needed.
In recent years, immunotherapy has been applied in cervical cancer. It can recruit the host immune system and kill cancer cells directly, thereby attenuating the recurrence and metastasis rates of cancer [6]. Nonetheless, there is significant heterogeneity in the therapeutic response and prognoses of cervical cancer patients with immunotherapy [7]. Only a subset of patients can derive benefit from immunotherapy. Thus, it is of great clinical significance to screen the sensitive patients. Extensive publications have reported that immune cell infiltration is closely related to the clinical therapeutic effect in various tumors [8,9]. Clinical efficacy of immunotherapies depends on a high density of preexistent tumor infiltrating immune cells [10]. Therefore, it is imperative for developing the suitable biomarkers to predict the immune cells infiltration and prognosis of cervical cancer patients.
In this research, we extracted and established a novel immune-related signature based on the analysis of cervical cancer genome and immunome. This signature can predict the immune cells infiltration and prognosis of cervical cancer simultaneously. It may contribute to better clinical personalized treatment and improve cervical cancer patients’ prognoses.
Material and Methods
DATA ACQUISITION:
The transcriptomic data of 306 cervical cancer samples from The Cancer Genome Atlas (TCGA) database (
CONSTRUCTION OF A NOVEL IMMUNE-RELATED SIGNATURE:
The R package “DESeq2” was used to screen the differentially expressed genes (DEGs) between cervical cancer tissues and normal cervical tissues [11]. The threshold criteria were set as |log2 (Fold Change) |>2 and adjusted P value <0.05. Volcano plots were created by using R package “ggplot2” (version 3.3.3). The list of immune-related genes (IRGs) was downloaded from Immunology Database and Analysis Portal (https://www.immport.org/home) [12]. A Venn diagram was generated to obtain the overlapping genes between differentially expressed genes (DEGs) and immune-related genes.
Univariate Cox proportional hazards regression analyses were performed to investigate the correlation between the expression of those overlapping genes and the overall survival (OS) of cervical cancer patients [13]. Genes with P value <0.05 were selected as candidate genes. Furthermore, Least Absolute Shrinkage and Selection Operator (LASSO) regression analysis was conducted with 10-fold cross-validation by using R package “glmnet” and “survival”. The genes with non-zero coefficients were selected as the hub immune-related genes to construct the novel signature. The risk score of the novel signature for each cervical cancer patient was calculated according to the expression level of each gene and corresponding LASSO regression coefficient. The cervical cancer patients in the training cohort, validation cohort, internal testing cohort, and external testing cohort were categorized into low-risk and high-risk groups based on the median value of risk scores.
VALIDATION OF THE NOVEL SIGNATURE:
To validate the predictive power of this novel immune-related signature, a Kaplan-Meier (K-M) survival curve was applied to estimate and compare the overall survival rates of low-risk and high-risk groups. The time-dependent receiver operating characteristic (ROC) curve illustrated the predictive sensitivity and specificity of this signature at 1, 3, and 5 years. All analyses described above were performed in 4 datasets: the training cohort, validation cohort, internal testing cohort, and external testing cohort. Univariate and multivariate Cox proportional hazards regression analyses were used to determine whether the novel signature was an independent prognostic factors for cervical cancer.
CONSTRUCTION AND VALIDATION OF A NOMOGRAM:
A nomogram was constructed with both the risk score of the novel signature and traditional clinical characteristics, including age, body mass index (BMI), clinical stage, M stage, N stage, and histological type. The calibration plots visually revealed the nomogram-predicted survival probability at 1, 3, and 5 years, which were achieved using R package “rms” (version 6.2-0) and “survival” (version 3.2-10) [13]. Decision curve analysis (DCA) was used to evaluate the clinical net benefit of different prediction models.
FUNCTIONAL ENRICHMENT ANALYSIS:
Functional enrichment analyses were performed to examine the biological functions of hub immune-related genes. Gene Ontology (GO) terms, including biological processes (BP), cellular components (CC), and molecular function (MF), were enriched and visualized via R package “GOplot”, “clusterProfiler”, and “ggplot2” [14,15].
ESTIMATION OF IMMUNE CELLS INFILTRATION:
The proportion of immune cells infiltration in each cervical cancer sample of TCGA datasets was estimated though a computational analytical tool CIBERSORT (http://cibersort.stanford.edu/) [16]. The relationship between the risk scores of novel signature and immune cells infiltration was investigated using single-sample GSEA (ssGSEA) method in R package “GSVA” (version 1.34.0) [17,18]. The fractions of immune cells between high-risk and low-risk groups were also compared.
VALIDATION OF THE HUB IMMUNE-RELATED GENES EXPRESSION:
To validate the expression of the 10 hub immune-related genes, we detected the mRNA and protein expression levels of these genes in cervical cancer tissues and normal tissues. The differential mRNA expression of 10 hub immune-related genes between cervical cancer tissues and normal tissues was investigated by RNA sequencing. The protein expression of these immune-related genes in cervical cancer tissues and normal tissues was detected by immunohistochemistry. The staining results were acquired from the Human Protein Atlas database (
STATISTICAL ANALYSIS:
All statistical analyses were processed with R software. Univariate and multivariate Cox regression analyses were conducted to determine the hazard ratios (HR) and 95% confidence intervals (CI) for events. The Mann-Whitney U test or
Results
IDENTIFICATION OF HUB IMMUNE-RELATED GENES:
Based on the differential expression analysis, there were 4338 differentially expressed genes presenting 2-fold or greater change between cervical cancer tissues and normal tissues. Therein, 2801 genes displayed up-regulated expression and 1537 genes displayed down-regulated expression (Figure 1A). Among 4338 differentially expressed genes, there were 298 genes having immunological-related functions (Figure 1B). Further univariate Cox proportional hazards regression analysis evaluated the prognostic values of these 298 genes. As shown in Supplementary Table 1, there were 38 genes significantly related with the overall survival of cervical cancer patients (P<0.05). Subsequently, LASSO regression modeling further minimized overfitting and selected 10 genes as the hub immune-related genes: TFRC, ZAP70, SPP1, CHIT1, CAMP, CSF2, TUBB3, LEPR, DLL4, and DES (Figure 1C). The forest plot showed that these hub immune-related genes have promise for use as prognostic biomarkers in cervical cancer (all P<0.05) (Figure 1D).
CONSTRUCTION OF A NOVEL IMMUNE-RELATED SIGNATURE:
The novel immune-related signature was constructed based on those 10 hub immune-related genes. The risk score of this novel immune-related signature was calculated using the expression level and corresponding correlation coefficient of each gene. Risk score=0.03749*TFRC-0.14105*ZAP70+0.05229*SPP1-0.06349*CHIT1-0.05564*CAMP+0.00302*CSF2+0.05782*TUBB3+0.06949*LEPR +0.13824*DLL4-0.03868*DES. The median value of risk scores for novel signature was identified as the cutoff point to categorize patients into a low-risk group and a high-risk group. In the training set, the cutoff value was 0.508 (Figure 2A). According to the cutoff, the patients in the high-risk group had significantly worse overall survival than those in the low-risk group (P<0.001). We evaluated the sensitivity and specificity of this novel immune-related signature for predicting the prognosis of patients with cervical cancer by using an ROC curve. The AUC of this signature reached 0.776 at 1 year, 0.747 at 3 years, and 0.778 at 5 years, which indicated a relatively high reliability of this novel signature. Taken together, this novel signature could predict the overall survival of cervical cancer patients.
VALIDATION OF THE NOVEL IMMUNE-RELATED SIGNATURE:
To validate the predictive accuracy and stability of this novel signature, the similar workflow was employed in another 3 datasets (Figure 2B–2D). The distribution of risk scores, survival statuses, and hub immune-related genes expression for these 3 datasets was exhibited. All cervical cancer patients were allocated into a high-risk or a low-risk group based on the cutoff point of the training cohort. For the validation cohort, Kaplan-Meier survival analysis revealed that patients in the high-risk group had worse prognosis than patients in the low-risk group (P<0.001). AUC values of 0.854, 0.857, and 0.853 were obtained for the 1-, 3-, and 5-year survival rates, respectively. Similar results were obtained in the internal and external testing sets. Significantly worse overall survival rates were observed in the cervical cancer patients with high-risk scores than those with low-risk scores (P<0.001 for the entire TCGA dataset and P=0.023 for GSE44001). The AUC values in 1, 3, and 5 years were all above 0.8 for the entire TCGA dataset and above 0.6 for GSE44001. Moreover, ROC curves demonstrated that the novel immune-related signature had better predictive ability than other independent genes, with the area under curve (AUC) of 0.739 (Supplementary Figure 1). Altogether, the novel signature was a highly shown to be a reliable biomarker for predicting the prognosis of cervical cancer patients.
PROGNOSTIC VALUE OF THE NOVEL IMMUNE-RELATED GENE SIGNATURE:
To determine whether the novel immune-related gene signature was an independent prognostic factor for cervical cancer patients, univariate and multivariate Cox regression analyses were performed in the entire TCGA cohort. As shown in Table 1, the immune-related gene signature, body mass index (BMI), clinical stage, M stage, and N stage were significantly associated with the overall survival of cervical cancer patients (all P<0.05). Further multivariate analysis revealed that the novel immune-related signature remained an independent prognostic indicator (HR=3.291, 95% CI=1.609–6.729, P=0.001). Then, a clinically quantitative prognostic model nomogram was developed based on the risk scores of this novel signature and some clinicopathological factors, including age, BMI, clinical stage, M stage, N stage, and histological type (Figure 3). The concordance index of this nomogram was 0.813 (0.782–0.843). Moreover, the calibration curves of this nomogram showed the excellent concordance between the predictive and actual 1-, 3-, and 5-year overall survival rates in patients with cervical cancer (Figure 4A–4C). The decision curve analysis (DCA) also indicated that the nomogram model, constructed by both novel signature and clinical characteristics, achieved the highest clinical net benefit across the entire range of reasonable threshold probabilities (Figure 4D). In addition, the risk score of the novel signature provided greater clinical net benefit than traditional clinical characteristics.
GENE ENRICHMENT ANALYSIS:
To further explore the biological function of the 10 hub immune-related genes, biological processes (BP), cellular components (CC), and molecular function (MF) enrichment analyses were carried out. The top 5 ranking pathways for each method are shown in Figure 5. The enriched pathways were mainly related to immunology, including T cell differentiation, T cell activation, negative thymic T cell selection, and immunological synapse.
CORRELATION BETWEEN THE IMMUNE-RELATED GENE SIGNATURE AND IMMUNE CELL INFILTRATION:
Since the most enriched pathways of the 10 hub immune-related genes were correlated with immunology, the association between the risk scores of this novel signature and immune cells infiltration was further explored. Spearman correlation tests indicated that the risk scores of this novel signature was negatively correlated with the infiltration of various immune cells, including T cells (r=−0.470, P<0.001), CD8 T cells (r=−0.294, P<0.001), T helper cells (r=−0.2331, P<0.001), Th1 cells (r=−0.306, P<0.001), TFH cells (r=−0.261, P<0.001), Treg cells (r=−0.398, P<0.001), B cells (r=−0.397, P<0.001), and DC cells (r=−0.279, P<0.001) (Figure 6A). Comparison of immune cells infiltrated fraction between low-risk and high-risk groups obtained similar results. The fraction of many immune cell subtypes in the high-risk group was significantly lower than that in the low-risk group (all P<0.001) (Figure 6B).
VALIDATION OF THE HUB GENES EXPRESSION IN CERVICAL NORMAL AND CANCER TISSUES:
All 10 hub immune-related genes were significantly differentially expressed in cervical normal and cancer tissues. Among them, the mRNA expression of TFRC, ZAP70, SPP1, CHIT1, CAMP, CSF2, and TUBB3 in cervical cancer tissues were significantly higher than that in normal cervical tissues, while LEPR, DLL4, and DES presented the opposite expression (Figure 7A). We also investigated the protein expression of TFRC, CAMP, ZAP70, TUBB3, and CHIT1 in cervical cancer tissues and normal tissues (Figure 7B). TFRC, CAMP, ZAP70, TUBB3, and CHIT1 proteins were highly expressed in cervical cancer tissues. These results were in accordance with the previous bioinformatics analysis based in TCGA database.
Discussion
Although the HPV vaccination and cervical cancer screening have been popularized, cervical cancer remains the second most common malignant cancer among females [19]. The therapeutic effect of metastatic and recurrent cervical cancer is very limited and the 5-year survival rate is still poor [20]. Thus, identifying effective predictive and therapeutic targets is a hot spot for current research. In recent years, many studies have shown that the expression of immune-related genes and the infiltration of immune cells were strongly associated with the clinical outcomes of a variety of cancers [21]. These findings provided new insights for the development of innovative prognostic markers and precision therapeutic targets for cervical cancer. Although previous studies reported various biomarkers, some studies only explored a single gene and some studies were only based on a single microarray dataset or database, thus limiting the credibility and comprehensiveness of the research.
In this study, through univariate Cox and LASSO regression analyses, we screened out 10 immune-related genes: TFRC, SPP1, CAMP, CSF2, TUBB3, ZAP70, CHIT1, LEPR, DLL4, and DES. With the rapid development of high-throughput sequencing, the direct relationship between some of these candidate genes and cervical cancer was explored in previous studies. Transferrin receptor (TFRC), a type II transmembrane glycoprotein, serves as an important iron transporter and participates in the onset and progression of many cancers, including cervical cancer [22]. Secreted phosphoprotein 1 (SPP1) is a secreted phosphoprotein that plays a critical role in the proliferation, migration, and chemotaxis of cancer cells [23]. Higher expression of SPP1 was strongly related to worse overall survival of cervical cancer patients [24]. Cathelicidin antimicrobial protein (CAMP) is mainly expressed in different host defense cells, including macrophages, neutrophils, and endothelial cells. A number of studies have found that CAMP conferred a tumorigenic effect in various cancers, which could promote tumor growth and invasion through recruiting immune cells [25,26]. Colony-stimulating factor 2 (CSF2) is an immune modulatory cytokine stimulating stem cells to produce granulocytes and monocytes. The roles of CSF2 in tumor progression vary in different cancers [27]. In cervical cancer, CSF2 upregulation may induce tumor-related leukocytosis and result in poor prognosis [28]. Tubulin-beta3 (TUBB3) is overexpressed in the more aggressive and resistant cancer phenotypes, which was closely related with the sensitivity of antimicrotubular chemotherapeutic agents [29–31]. Our study produced similar results. The expression of TFRC, SPP1, CAMP, CSF2, TUBB3, ZAP70, and CHIT1 in cervical cancer tissues were significantly higher than that in normal cervical tissue. The opposite results were observed in the expression levels of LEPR, DLL4, and DES. All of these 10 immune-related genes showed diagnostic and prognostic value in cervical cancer.
Nevertheless, a single biomarker may omit large amounts of information and it is difficult to evaluate the prognosis comprehensively. Therefore, a novel predictive signature incorporating these 10 immune-related genes was constructed in this study. Compared with those single biomarker, this signature provided a more reliable and more comprehensive assessment in cervical cancer. It was proved that the risk score of this novel signature could be an independent prognostic predictor with high sensitivity and specificity. The signature successfully classified patients into high-risk and low-risk subgroups with different survival outcomes. Patients with high risk scores exhibited worse overall survival than those with low risk scores. In addition, the risk score of this novel signature presented higher net benefit than traditional clinical characteristics, including age, BMI, clinical stage, distant metastatic status, lymph node status, and histological type. The model generated by both clinical characteristics and this novel signature was the best in predicting the overall survival of cervical cancer patients. Furthermore, the model had the ability to provide a quantifiable assessment of the survival risk per individual patient. This is of great significance in clinical application in terms of individual treatment of cervical cancer.
Further gene ontology enrichment analyses indicated that these 10 genes were significantly enriched in immune-related pathways, including T cell differentiation, T cell activation, and immunological synapse. Therefore, the association between the novel signature and immune cells infiltration in cervical cancer was explored in our study. The results showed that the risk scores of this novel signature had significant negative correlations with the infiltrated statuses of most immune cell types, especially T cell subsets. Compared with the low-risk group, the proportions of infiltrated immune cells were significantly lower in the high-risk group. Compelling evidence has demonstrated that the infiltration of immune cells has an important effect on patients’ prognoses in a number of human malignancies [32–34]. T cells in particular are considered a major element of the tumor microenvironment. Intratumoral T cells display dysfunctional states in mouse models and cancer patients, which is shaped by multifaceted distinct immunosuppressive mechanisms [35]. A major mechanism is the increased expression of inhibitory receptors on T cell surfaces, such as programmed cell death-1 (PD-1), T cell immunoglobulin mucin receptor 3 (TIM3), lymphocyte activation gene 3 (LAG3), and cytotoxic T lymphocyte-associated protein 4 (CTLA4) [36–38]. Another is the reduced production of cytokines for T cells activation, such as interleukin-2 (IL-2), tumor necrosis factor (TNF), β-chemokines, and interferon-γ (IFNγ) [39,40]. Low infiltration of intratumoral T cells predicted poor overall survival and recurrence-free survival [41]. Our findings are consistent with these previous studies. Hence, it is reasonable to infer that our novel signature has potential for use as a predictive and therapeutic target for immune escape in cervical cancer.
There were several limitations in the present study. Firstly, this was a retrospective study. More multicenter prospective clinical cohort studies are needed to validate the prognostic value of our novel signature. Secondly, a small portion of data in the TCGA cohort was missing, which potentially hindered a more comprehensive analysis. Last but not least, the underlying biological mechanisms of the novel signature and immune cell infiltration need to be investigated by in vivo and in vitro experiments.
Conclusions
This novel signature incorporating 10 immune-related genes could provide a more comprehensive assessment in cervical cancer. It would be a valuable biomarker to individualize the risk assessment and predict the prognosis and immune infiltration for each patient. In the era of precision medicine, this novel signature may hold great promise to assist treatment selection and prognostic stratification in cervical cancer.
Figures
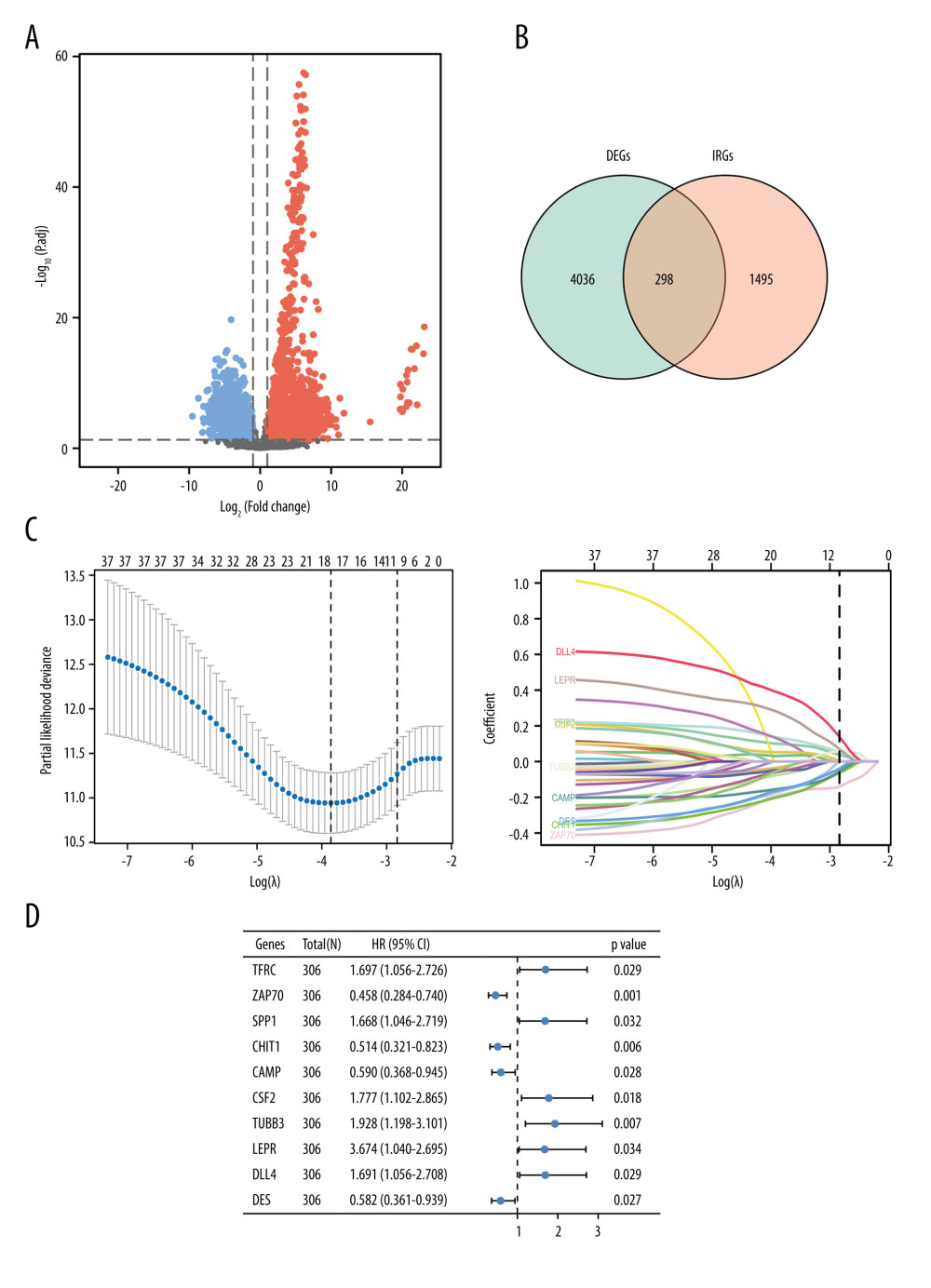 Figure 1. Identification of immune-related genes in cervical cancer.(A) Volcano plot of the differentially expressed genes between cervical cancer and normal tissue. Red points represented the significantly up-regulated genes, while blue points represent the significantly down-regulated genes (adjusted P<0.05). (B) Venn diagram to identify the overlapping genes between differentially expressed genes and immune-related genes. (C) Cross-validation plot for tuning the penalty parameter lambda in the LASSO model. LASSO coefficient profile plot of hub immune-related genes. (D) Forest plot of univariate Cox proportional hazards regression analysis for 10 hub immune-related genes expression and overall survival.
Figure 1. Identification of immune-related genes in cervical cancer.(A) Volcano plot of the differentially expressed genes between cervical cancer and normal tissue. Red points represented the significantly up-regulated genes, while blue points represent the significantly down-regulated genes (adjusted P<0.05). (B) Venn diagram to identify the overlapping genes between differentially expressed genes and immune-related genes. (C) Cross-validation plot for tuning the penalty parameter lambda in the LASSO model. LASSO coefficient profile plot of hub immune-related genes. (D) Forest plot of univariate Cox proportional hazards regression analysis for 10 hub immune-related genes expression and overall survival. 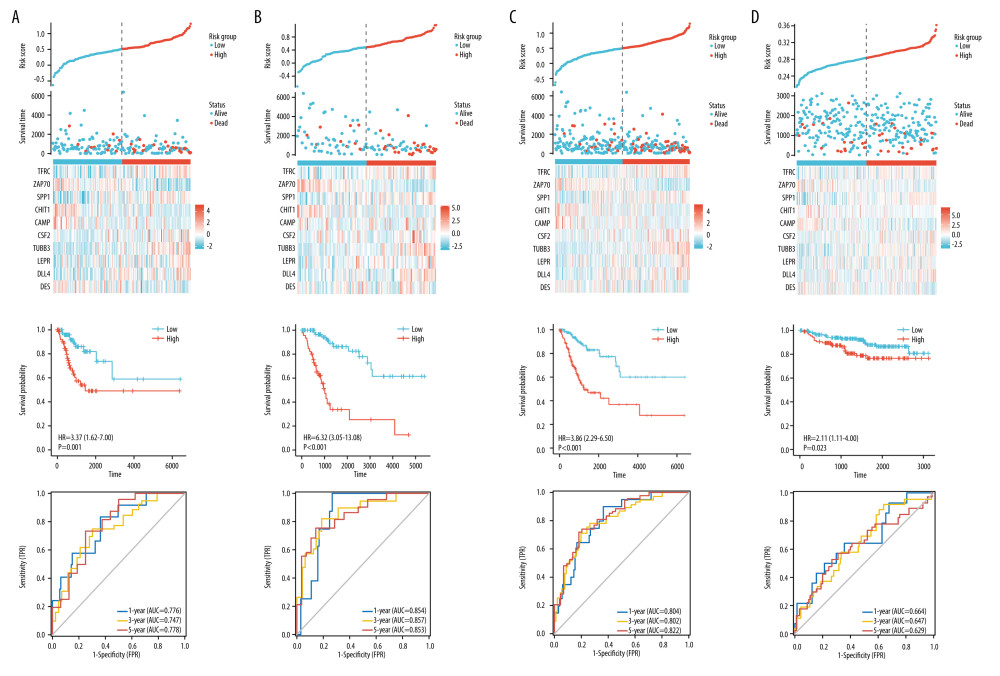 Figure 2. Construction and validation of the novel signature.(A) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in TCGA training set. Kaplan-Meier curves for TCGA training set. Time-dependent ROC curves of risk scores for TCGA training set. (B) The distributions of risk scores, overall survival statuses, and expression profiles of 10 immune-related genes in TCGA validation set. Kaplan-Meier curves for TCGA validation set. Time-dependent ROC curves of risk scores for TCGA validation set. (C) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in internal testing set. Kaplan-Meier curves for internal testing set. Time-dependent ROC curves of risk scores for internal testing set. (D) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in the external testing set. Kaplan-Meier curves for external testing set. Time-dependent ROC curves of risk scores for external testing set.
Figure 2. Construction and validation of the novel signature.(A) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in TCGA training set. Kaplan-Meier curves for TCGA training set. Time-dependent ROC curves of risk scores for TCGA training set. (B) The distributions of risk scores, overall survival statuses, and expression profiles of 10 immune-related genes in TCGA validation set. Kaplan-Meier curves for TCGA validation set. Time-dependent ROC curves of risk scores for TCGA validation set. (C) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in internal testing set. Kaplan-Meier curves for internal testing set. Time-dependent ROC curves of risk scores for internal testing set. (D) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in the external testing set. Kaplan-Meier curves for external testing set. Time-dependent ROC curves of risk scores for external testing set. 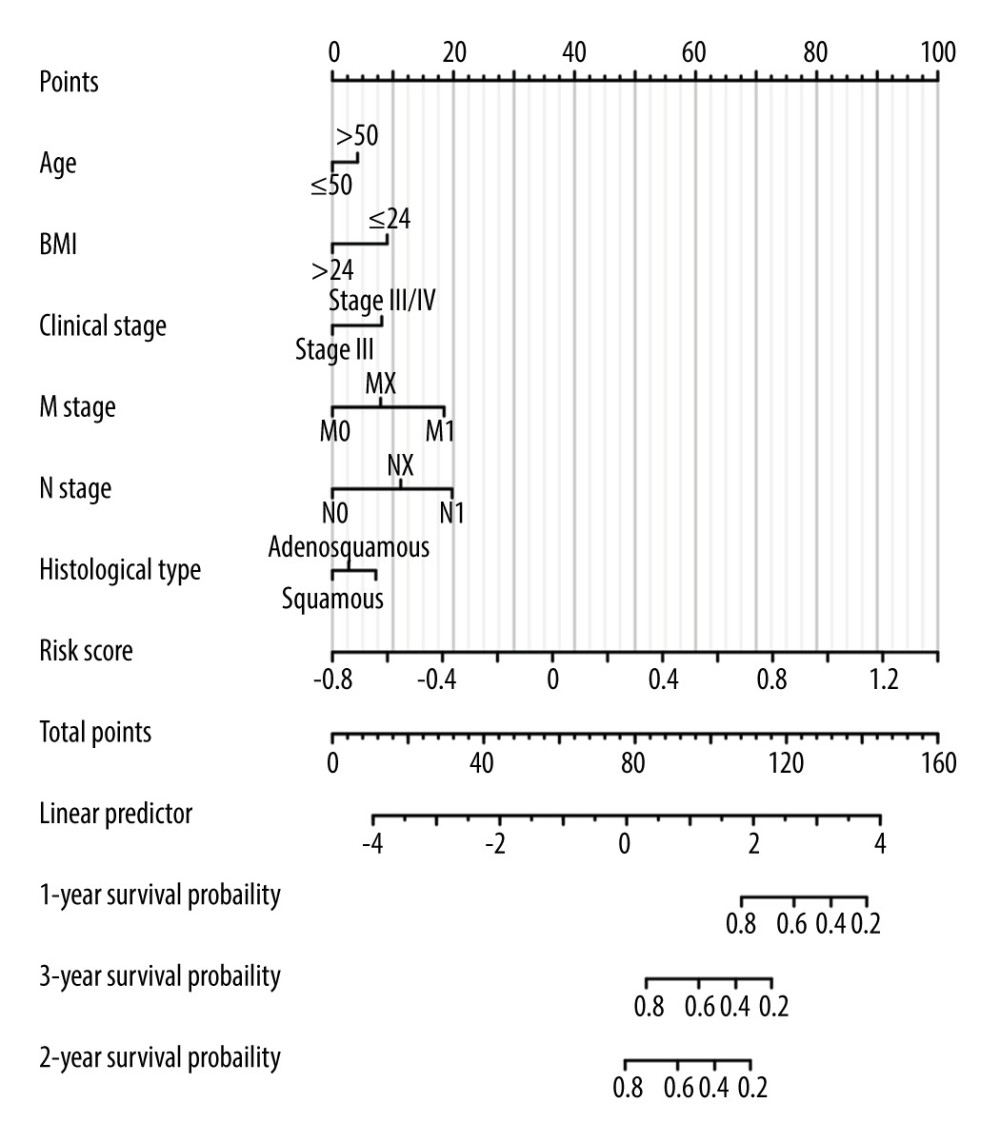 Figure 3. Nomogram for predicting the 1-, 3-, and 5-year overall survival rates.
Figure 3. Nomogram for predicting the 1-, 3-, and 5-year overall survival rates. 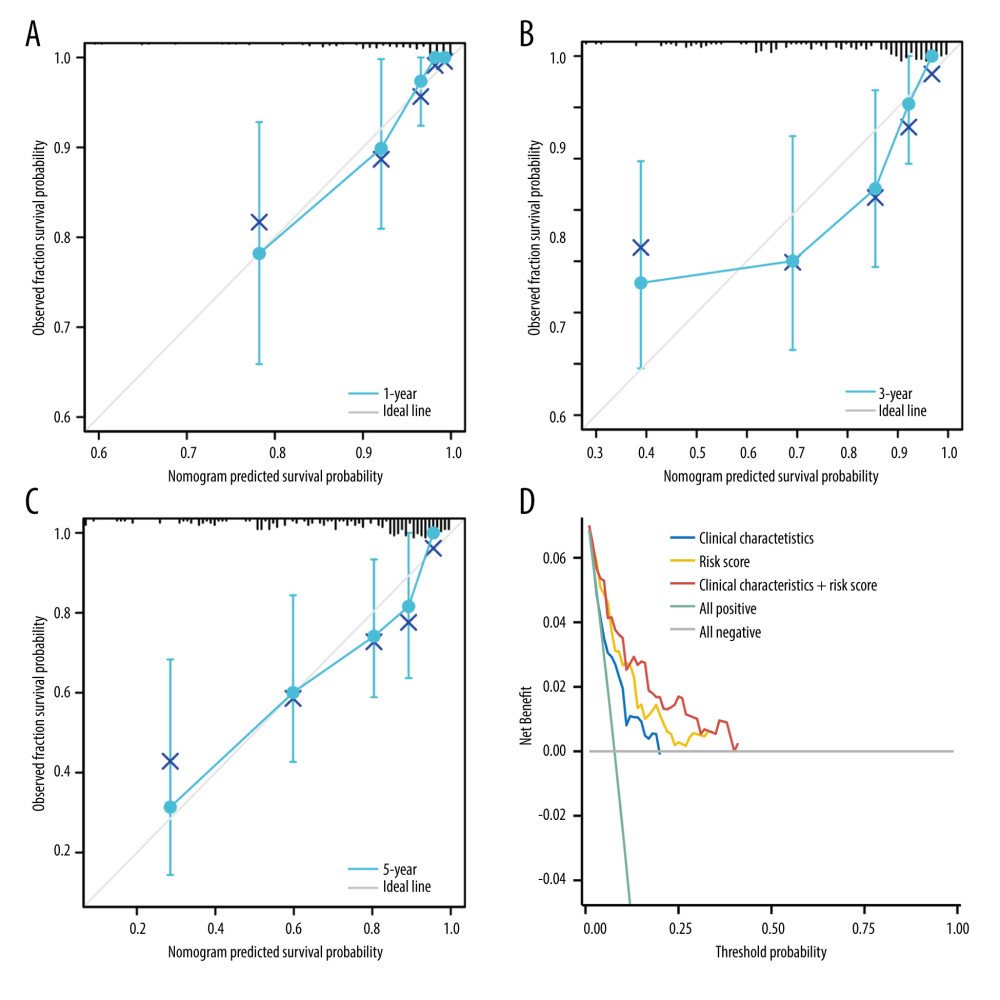 Figure 4. The predictive model for overall survival in cervical cancer.(A) Calibration plot for the nomogram predicting 1-year overall survival. (B) Calibration plot for the nomogram predicting 3-year overall survival. (C) Calibration plot for the nomogram predicting 5-year overall survival. (D) Decision curve analysis (DCA) showed the clinical net benefits of different prediction models.
Figure 4. The predictive model for overall survival in cervical cancer.(A) Calibration plot for the nomogram predicting 1-year overall survival. (B) Calibration plot for the nomogram predicting 3-year overall survival. (C) Calibration plot for the nomogram predicting 5-year overall survival. (D) Decision curve analysis (DCA) showed the clinical net benefits of different prediction models. 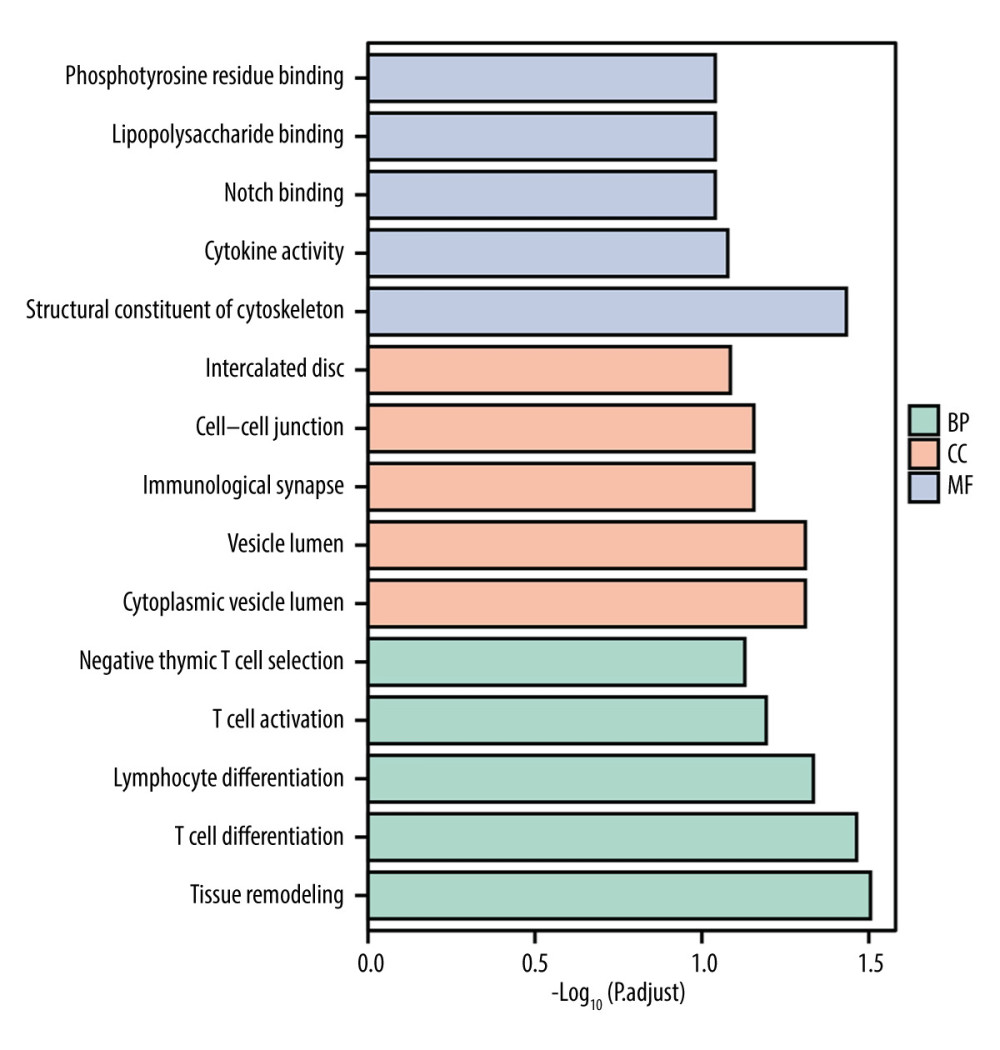 Figure 5. Gene Ontology terms (including BP, MF, and CC) of immune-related genes in cervical cancer.
Figure 5. Gene Ontology terms (including BP, MF, and CC) of immune-related genes in cervical cancer. 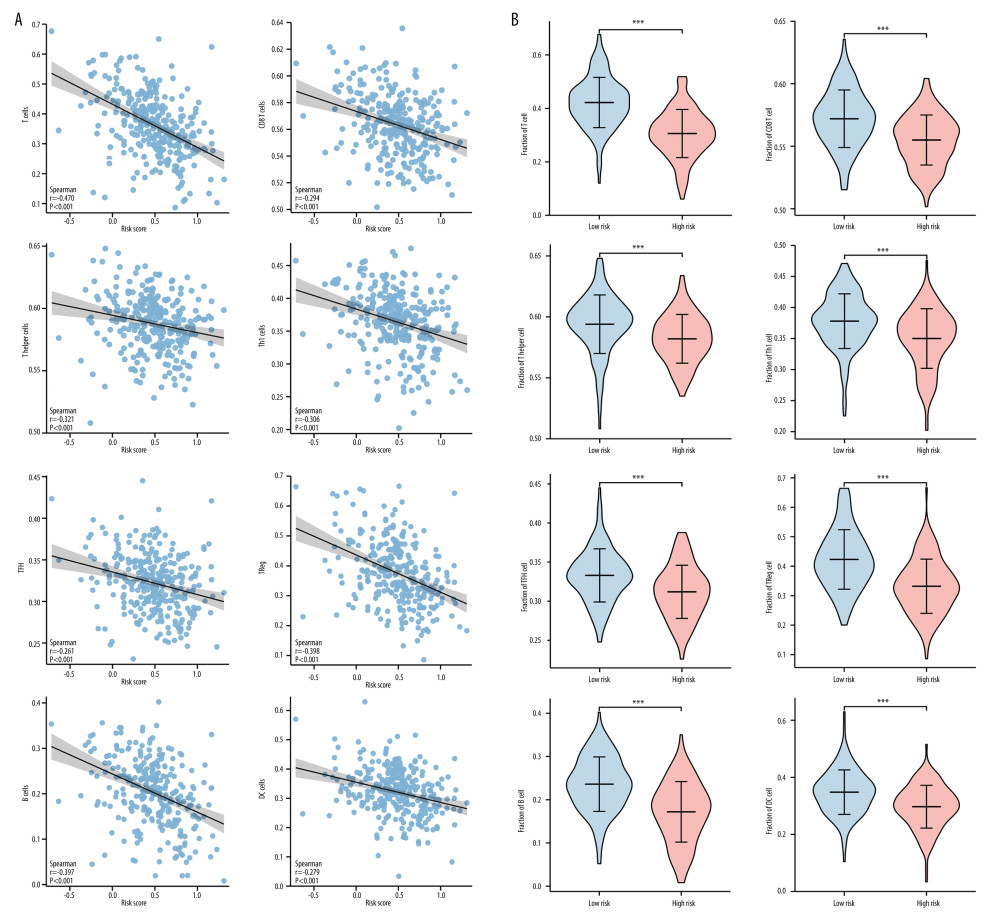 Figure 6. Correlation between the novel signature and immune cell infiltration in cervical cancer.(A) Correlation analyses between the risk scores and infiltrated immune cells. (B) Comparison of the fraction of immune cells between high-risk and low-risk groups.
Figure 6. Correlation between the novel signature and immune cell infiltration in cervical cancer.(A) Correlation analyses between the risk scores and infiltrated immune cells. (B) Comparison of the fraction of immune cells between high-risk and low-risk groups. 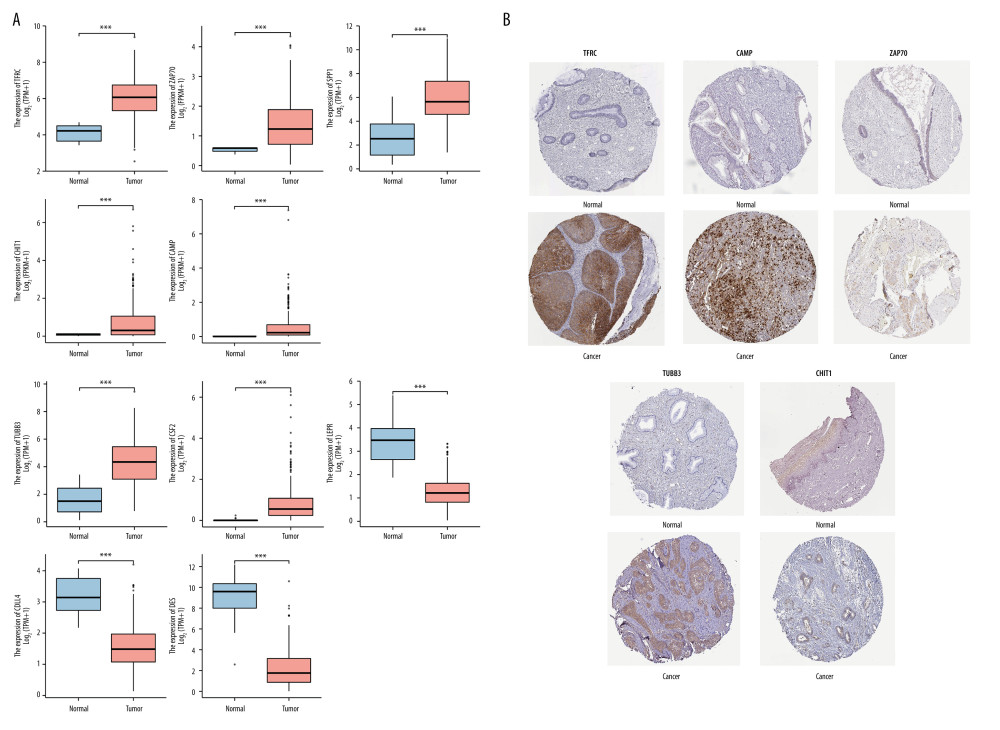 Figure 7. The expression levels of hub immune-related genes in cervical normal and cancer tissues.(A) The mRNA expression of 10 hub immune-related genes in cervical normal and cancer tissues. (B) The immunostaining of TFRC, CAMP, ZAP70, TUBB3, and CHIT1 in cervical cancer tissues and normal tissues.
Figure 7. The expression levels of hub immune-related genes in cervical normal and cancer tissues.(A) The mRNA expression of 10 hub immune-related genes in cervical normal and cancer tissues. (B) The immunostaining of TFRC, CAMP, ZAP70, TUBB3, and CHIT1 in cervical cancer tissues and normal tissues. References
1. Sung H, Ferlay J, Siegel RL, Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2021; 71(3); 209-49
2. Randall TC, Sauvaget C, Muwonge R, Worthy of further consideration: An updated meta-analysis to address the feasibility, acceptability, safety and efficacy of thermal ablation in the treatment of cervical cancer precursor lesions: Prev Med, 2019; 118; 81-91
3. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019: Cancer J Clin, 2019; 69(1); 7-34
4. Sankaranarayanan R, Basu P, Kaur P, Current status of human papillomavirus vaccination in India’s cervical cancer prevention efforts: Lancet Oncol, 2019; 20(11); e637-e44
5. Arbyn M, Weiderpass E, Bruni L, Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis: Lancet Glob Health, 2020; 8(2); e191-e203
6. Wendel Naumann R, Leath CA, Advances in immunotherapy for cervical cancer: Curr Opin Oncol, 2020; 32(5); 481-87
7. Mauricio D, Zeybek B, Tymon-Rosario J, Immunotherapy in cervical cancer: Curr Oncol Rep, 2021; 23(6); 61
8. Zhang Y, Zhang Z, The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications: Cellul Mol Immunol, 2020; 17(8); 807-21
9. Gentles AJ, Newman AM, Liu CL, The prognostic landscape of genes and infiltrating immune cells across human cancers: Nat Med, 2015; 21(8); 938-45
10. Naing A, Infante JR, Papadopoulos KP, PEGylated IL-10 (Pegilodecakin) induces systemic immune activation, CD8(+) T cell invigoration and polyclonal T cell expansion in cancer patients: Cancer Cell, 2018; 34(5); 775-91e3
11. Love MI, Huber W, Anders S, Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2: Genome Biol, 2014; 15(12); 550
12. Weinstein JN, Collisson EA, Mills GB, The Cancer Genome Atlas Pan-Cancer analysis project: Nat Genet, 2013; 45(10); 1113-20
13. Liu J, Lichtenberg T, Hoadley KA, An integrated TCGA Pan-Cancer clinical data resource to drive high-quality survival outcome analytics: Cell, 2018; 173(2); 400-16e11
14. Walter W, Sánchez-Cabo F, Ricote M, GOplot: An R package for visually combining expression data with functional analysis: Bioinformatics, 2015; 31(17); 2912-14
15. Yu G, Wang LG, Han Y, He QY, clusterProfiler: An R package for comparing biological themes among gene clusters: OMICS, 2012; 16(5); 284-87
16. Newman AM, Liu CL, Green MR, Robust enumeration of cell subsets from tissue expression profiles: Nat Methods, 2015; 12(5); 453-57
17. Hänzelmann S, Castelo R, Guinney J, GSVA: Gene set variation analysis for microarray and RNA-seq data: BMC Bioinformatics, 2013; 14; 7
18. Bindea G, Mlecnik B, Tosolini M, Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer: Immunity, 2013; 39(4); 782-95
19. Das M, WHO launches strategy to accelerate elimination of cervical cancer: Lancet Oncol, 2021; 22(1); 20-21
20. Burd EM, Human papillomavirus and cervical cancer: Clin Microbiol Rev, 2003; 16(1); 1-17
21. Hinshaw DC, Shevde LA, The tumor microenvironment innately modulates cancer progression: Cancer Res, 2019; 79(18); 4557-66
22. Xu X, Liu T, Wu J, Transferrin receptor-involved HIF-1 signaling pathway in cervical cancer: Cancer gene Ther, 2019; 26(11–12); 356-65
23. Su X, Xu BH, Zhou DL, Polymorphisms in matricellular SPP1 and SPARC contribute to susceptibility to papillary thyroid cancer: Genomics, 2020; 112(6); 4959-67
24. Deepti P, Pasha A, Kumbhakar DV, Overexpression of secreted Phosphoprotein 1 (SPP1) predicts poor survival in HPV positive cervical cancer: Gene, 2022; 824; 146381
25. Chen J, Shin VY, Ho JC, Functional implications of cathelicidin antimicrobial protein in breast cancer and tumor-associated macrophage microenvironment: Biomolecules, 2020; 10(5); 688
26. Chow JY, Li ZJ, Wu WK, Cho CH, Cathelicidin a potential therapeutic peptide for gastrointestinal inflammation and cancer: World J Gastroenterol, 2013; 19(18); 2731-35
27. Hong IS, Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types: Exp Mol Med, 2016; 48(7); e242
28. Kim KH, Sim NS, Chang JS, Kim YB, Tumor immune microenvironment in cancer patients with leukocytosis: Cancer Immunol Immunother, 2020; 69(7); 1265-77
29. Namekawa T, Ikeda K, Horie-Inoue K, ALDH1A1 in patient-derived bladder cancer spheroids activates retinoic acid signaling leading to TUBB3 overexpression and tumor progression: Int J Cancer, 2020; 146(4); 1099-113
30. Liu Z, Li S, Dong J, Miao Y, TUBB3 promotes growth and invasion of gallbladder cancer cells by Akt/mTOR signal pathway: J Environ Pathol Toxicol Oncol, 2021; 40(2); 23-33
31. Sekino Y, Han X, Kawaguchi T, TUBB3 reverses resistance to docetaxel and cabazitaxel in prostate cancer: Int J Mol Sci, 2019; 20(16); 3936
32. Thommen DS, Schumacher TN, T cell dysfunction in cancer: Cancer Cell, 2018; 33(4); 547-62
33. Mami-Chouaib F, Blanc C, Corgnac S, Resident memory T cells, critical components in tumor immunology: J Immunother Cancer, 2018; 6(1); 87
34. Liu Z, Zhou Q, Wang Z, Intratumoral TIGIT(+) CD8(+) T-cell infiltration determines poor prognosis and immune evasion in patients with muscle-invasive bladder cancer: J Immunother Cancer, 2020; 8(2); e000978
35. Cimen Bozkus C, Roudko V, Finnigan JP, Immune checkpoint blockade enhances shared neoantigen-induced T-cell immunity directed against mutated calreticulin in myeloproliferative neoplasms: Cancer Discov, 2019; 9(9); 1192-207
36. Bengsch B, Johnson AL, Kurachi M, Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion: Immunity, 2016; 45(2); 358-73
37. van der Leun AM, Thommen DS, Schumacher TN, CD8(+) T cell states in human cancer: Insights from single-cell analysis: Nat Rev Cancer, 2020; 20(4); 218-32
38. Chihara N, Madi A, Kondo T, Induction and transcriptional regulation of the co-inhibitory gene module in T cells: Nature, 2018; 558(7710); 454-59
39. Wherry EJ, Ha S-J, Kaech SM, Molecular signature of CD8+ T cell exhaustion during chronic viral inf ection: Immunity, 2007; 27(4); 670-84
40. Wherry EJ, Kurachi M, Molecular and cellular insights into T cell exhaustion: Nat Rev Immunol, 2015; 15(8); 486-99
41. Litwin TR, Irvin SR, Chornock RL, Infiltrating T-cell markers in cervical carcinogenesis: A systematic review and meta-analysis: Br J Ccancer, 2021; 124(4); 831-41
Figures
 Figure 1. Identification of immune-related genes in cervical cancer.(A) Volcano plot of the differentially expressed genes between cervical cancer and normal tissue. Red points represented the significantly up-regulated genes, while blue points represent the significantly down-regulated genes (adjusted P<0.05). (B) Venn diagram to identify the overlapping genes between differentially expressed genes and immune-related genes. (C) Cross-validation plot for tuning the penalty parameter lambda in the LASSO model. LASSO coefficient profile plot of hub immune-related genes. (D) Forest plot of univariate Cox proportional hazards regression analysis for 10 hub immune-related genes expression and overall survival.
Figure 1. Identification of immune-related genes in cervical cancer.(A) Volcano plot of the differentially expressed genes between cervical cancer and normal tissue. Red points represented the significantly up-regulated genes, while blue points represent the significantly down-regulated genes (adjusted P<0.05). (B) Venn diagram to identify the overlapping genes between differentially expressed genes and immune-related genes. (C) Cross-validation plot for tuning the penalty parameter lambda in the LASSO model. LASSO coefficient profile plot of hub immune-related genes. (D) Forest plot of univariate Cox proportional hazards regression analysis for 10 hub immune-related genes expression and overall survival. Figure 2. Construction and validation of the novel signature.(A) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in TCGA training set. Kaplan-Meier curves for TCGA training set. Time-dependent ROC curves of risk scores for TCGA training set. (B) The distributions of risk scores, overall survival statuses, and expression profiles of 10 immune-related genes in TCGA validation set. Kaplan-Meier curves for TCGA validation set. Time-dependent ROC curves of risk scores for TCGA validation set. (C) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in internal testing set. Kaplan-Meier curves for internal testing set. Time-dependent ROC curves of risk scores for internal testing set. (D) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in the external testing set. Kaplan-Meier curves for external testing set. Time-dependent ROC curves of risk scores for external testing set.
Figure 2. Construction and validation of the novel signature.(A) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in TCGA training set. Kaplan-Meier curves for TCGA training set. Time-dependent ROC curves of risk scores for TCGA training set. (B) The distributions of risk scores, overall survival statuses, and expression profiles of 10 immune-related genes in TCGA validation set. Kaplan-Meier curves for TCGA validation set. Time-dependent ROC curves of risk scores for TCGA validation set. (C) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in internal testing set. Kaplan-Meier curves for internal testing set. Time-dependent ROC curves of risk scores for internal testing set. (D) The distributions of risk scores, overall survival statuses and expression profiles of 10 immune-related genes in the external testing set. Kaplan-Meier curves for external testing set. Time-dependent ROC curves of risk scores for external testing set. Figure 3. Nomogram for predicting the 1-, 3-, and 5-year overall survival rates.
Figure 3. Nomogram for predicting the 1-, 3-, and 5-year overall survival rates. Figure 4. The predictive model for overall survival in cervical cancer.(A) Calibration plot for the nomogram predicting 1-year overall survival. (B) Calibration plot for the nomogram predicting 3-year overall survival. (C) Calibration plot for the nomogram predicting 5-year overall survival. (D) Decision curve analysis (DCA) showed the clinical net benefits of different prediction models.
Figure 4. The predictive model for overall survival in cervical cancer.(A) Calibration plot for the nomogram predicting 1-year overall survival. (B) Calibration plot for the nomogram predicting 3-year overall survival. (C) Calibration plot for the nomogram predicting 5-year overall survival. (D) Decision curve analysis (DCA) showed the clinical net benefits of different prediction models. Figure 5. Gene Ontology terms (including BP, MF, and CC) of immune-related genes in cervical cancer.
Figure 5. Gene Ontology terms (including BP, MF, and CC) of immune-related genes in cervical cancer. Figure 6. Correlation between the novel signature and immune cell infiltration in cervical cancer.(A) Correlation analyses between the risk scores and infiltrated immune cells. (B) Comparison of the fraction of immune cells between high-risk and low-risk groups.
Figure 6. Correlation between the novel signature and immune cell infiltration in cervical cancer.(A) Correlation analyses between the risk scores and infiltrated immune cells. (B) Comparison of the fraction of immune cells between high-risk and low-risk groups. Figure 7. The expression levels of hub immune-related genes in cervical normal and cancer tissues.(A) The mRNA expression of 10 hub immune-related genes in cervical normal and cancer tissues. (B) The immunostaining of TFRC, CAMP, ZAP70, TUBB3, and CHIT1 in cervical cancer tissues and normal tissues.
Figure 7. The expression levels of hub immune-related genes in cervical normal and cancer tissues.(A) The mRNA expression of 10 hub immune-related genes in cervical normal and cancer tissues. (B) The immunostaining of TFRC, CAMP, ZAP70, TUBB3, and CHIT1 in cervical cancer tissues and normal tissues. Tables
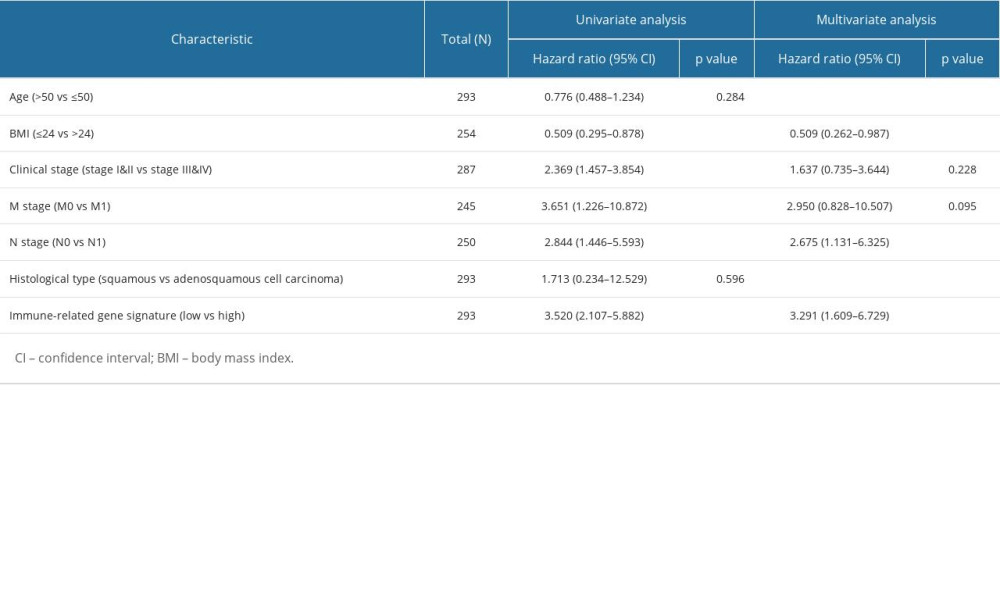 Table 1. Univariate and multivariate Cox regression analyses for prognostic factors and overall survival of cervical cancer.
Table 1. Univariate and multivariate Cox regression analyses for prognostic factors and overall survival of cervical cancer. Table 1. Univariate and multivariate Cox regression analyses for prognostic factors and overall survival of cervical cancer.
Table 1. Univariate and multivariate Cox regression analyses for prognostic factors and overall survival of cervical cancer. Supplementary Table 1. Univariate Cox regression analyses for 298 genes and overall survival of cervical cancer.
Supplementary Table 1. Univariate Cox regression analyses for 298 genes and overall survival of cervical cancer. In Press
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








