29 September 2022: Clinical Research
Postoperative Dynamic of Leptin and Fibroblast Growth Factor 21 in 123 Patients Recovering from Cardiac Surgery
Da Liu1BCE, Danyal Ghani2BCE, Wilson Y. Szeto3ABE, Krzysztof Laudanski456ABCDEFG*DOI: 10.12659/MSM.937652
Med Sci Monit 2022; 28:e937652
Abstract
BACKGROUND: Cardiac surgery triggers acute changes in serum leptin and fibroblast growth factor 21 (FGF-21). Considering their pleiotropic role in inflammation and abnormal glucose metabolism, perseverance of their abnormal serum level can have a long-term impact on recovery and end-organ failures. Long-term dynamics after cardiac surgery are unknown.
MATERIAL AND METHODS: Serum was collected from 123 patients before cardiac surgery (tbaseline) and 24 h (t24h), 7 days (t7d), and 3 months (t3m) later. Also, interleukin 6 (IL-6) and C-reactive protein (CRP) assessed nonspecific inflammatory responses. Neurodegeneration was gauged with serum amyloid β1-40 and β1-42. Demographic and clinical information, including disposition at 28 days and t3m from admission, were collected.
RESULTS: Serum leptin increased at t24h (leptinbaseline=613+747.9 vs leptin24h=768±718.1; P=0.0083) and decreased at t7d (leptin7d=499.5±540.2; P=0.043). FGF-21 levels increased at t24h and t7d. Cytokines normalized by t3m. Presurgical leptin levels were higher in Asians and were the primary determinant of postoperative leptin changes. Leptin levels were most elevated in patients undergoing aortic valve and arch surgery; the perioperative increase was significant only in patients with mitral valve surgery. Leptin and FGF-21 did not correlate with markers of general inflammation (CRP, IL-6), which partially resolved after t3m. Amyloid β1-42 at t3m correlated with leptin peak at t24h. Low prehospital FGF-21 level correlated with the incidence of perioperative stroke; postoperative FGF-21 correlated with discharge to facility vs home.
CONCLUSIONS: Leptin and FGF-21 evolve independently from the inflammatory response in the aftermath of cardiac surgery and correlate with cardiac remodeling and neurodegeneration markers.
Keywords: C-Reactive Protein, Critical Care Outcomes, Fibroblast Growth Factor 21, Leptin, Survivors, Thoracic Surgery, Treatment Outcome, Cardiac Surgical Procedures, Cytokines, Fibroblast Growth Factors, Glucose, Humans, Inflammation, Interleukin-6
Background
Severe stress results in acute abnormalities of metabolic regulation of carbohydrates and lipids, which are detrimental to mortality and morbidity [1–3]. Patients undergoing coronary artery bypass grafting (CABG) seem particularly vulnerable. Prolonged abnormalities in carbohydrate metabolism can sustain ongoing sympathetic over-activation, inflammation, and changes in metabolome [1,2]. These conditions can accelerate arteriosclerosis and sustain sterile neuroinflammation, leading to premature neurodegeneration, denying patients the full long-term benefit of surgery [3,4].
Leptin is a critical hormone intersecting carbohydrate and lipid metabolism, with pleiotropic immunological activities [5]. Abnormal regulation of this hormone leads to the myocardium’s susceptibility to ischemia, inflammation persistence, and hyperglycemia [2,5–9]. Furthermore, leptin regulates satiety, which can be critical for maintaining an optimal body mass index (BMI) after surgery. Prior work demonstrated that leptin serum levels are diminished at 30 days after orthopedic surgery or cardiopulmonary bypass surgery [7]. Another study demonstrated an initial increase that was followed by diminishing levels, but the observation period was less than 24 h, and the study focused on the adult population [5]. The perisurgical increase in leptin levels is limited to serum and adipose tissue [10]. Changes in leptin levels can impact response to shock and infection susceptibility [8,11,12]. Consequently, these data are well aligned with the observation that preoperative abnormal leptin levels are correlated with a composite risk of the adverse cardiovascular level after cardiac surgery [13]. Fibroblast growth factor 21 (FGF-21) has a similar function, driving behavior aimed at increased carbohydrate intake. Combined, the abnormal regulation of these hormones can lead to hyperglycemia, persistent sterile inflammation in the kidney and brain, and susceptibility of the myocardium to ischemia, culminating in unfavorable long-term neurocognitive sequela [1,2]. All these effects mitigate the beneficial effects of heart surgery. It has been suggested that markers may be involved in unfavorable outcomes after cardiac surgery, including cognitive decline and unfavorable cardiac remodeling [14,15]. However, the longitudinal dynamics of the markers after major surgery are unknown. Although the hormones are released during acute stress, it is unclear if their stress-induced secretion outlasts the acute recovery from surgical stress, thereby contributing to long-term recovery from surgery.
This study aimed to investigate the longitudinal dynamics of leptin and FGF-21 in patients recovering from cardiac surgery. We hypothesized that leptin and FGF-21 abnormalities would be limited to the acute inflammatory response and follow serum interleukin 6 (IL-6) and C-reactive protein (CRP). To assess the clinical impact, we assessed serum levels of amyloids β1–40 and amyloids β1–42. In addition, considering hormone function, we investigated the effects of leptin in patients with diabetes or a higher BMI.
Material and Methods
PATIENT ENROLLMENT:
Our study protocol was approved by the Institutional Review Board (IRB) of the University of Pennsylvania and was performed according to the guidelines of the 2003 Helsinki Declaration (#815686; approved March 02, 2020).
All patients scheduled for elective heart surgery were approached for consent. We excluded patients with pre-existing immunological aberrancies who were on immunosuppressant medications in the last 6 months (oral or intravenous prednisone more than 5mg daily; αTNFα, αIL-6, αIL-3, and αCD20 antibody therapy; immunoglobulin, plasmapheresis, methotrexate, and chemotherapy). The study did not include patients with inherited and known dyslipidemias and those who had undergone transplantation.
The demographic characteristics of patients is presented in Table 1.
SAMPLE PROCESSING:
Upon patient consent, serum was isolated and stored at −80°C. Blood was collected at 4 times as follows: The baseline sample (tbaseline) was collected before or shortly after arterial or central line placement. The second sample was collected 24 h after the first sample (t24h), during the patient’s stay in the Intensive Care Unit (ICU). The third sample was obtained at the patient’s discharge from the hospital or 7 days after the tbaseline sample (t7d). The last sample was collected no sooner than 3 months after surgery (t3m) but no later than 4 months after. The 4 samples represents baseline, acute stress response, convalescence, and medium-term recovery, respectively.
CLINICAL DATA:
The electronic medical records were used to collect the demographic and clinical data for all enrolled patients. The patients self-determined their race and ethnicity. Several variables regarding the duration of surgery and anesthesia were collected from the medical records retrospectively. Preoperative hemoglobin A1c (HbA1c) and lipid profiles were collected from routine preoperative laboratory tests, when available. The Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were calculated within 1 h and t24h after admission to the ICU. The burden of chronic disease was calculated using the Charlson Comorbidity Index [16,17]. The severity of illness during the ICU stay was determined using the Marshalls Organ Dysfunction Score [18]. Survival was determined at 28 days and t3m from admission.
ASSESSMENT OF BIOMARKERS:
Leptin and FGF-21 were anlayzed using enzyme-linked immunosorbent assay, according to the manufacturer instructions (BioLegend, San Diego, CA, USA). In addition, inflammatory markers (IL-6, CRP) and neurodegeneration markers (amyloid β1–40, amyloid β1–42) were analyzed using multiplex technology (Theromofisher, Waltham, MA, USA) on a MagPix machine (Luminex, Austin, TX, USA) [19].
STATISTICAL ANALYSIS:
The normality of distribution of studied variables was determined using the Kolmogorov test and descriptive variables. Data were are presented as mean±standard deviation or median and interquartile ranges. The data were compared using the
Results
PATIENT CHARACTERISTICS AND BASELINE LEVELS OF LEPTIN AND FGF-21:
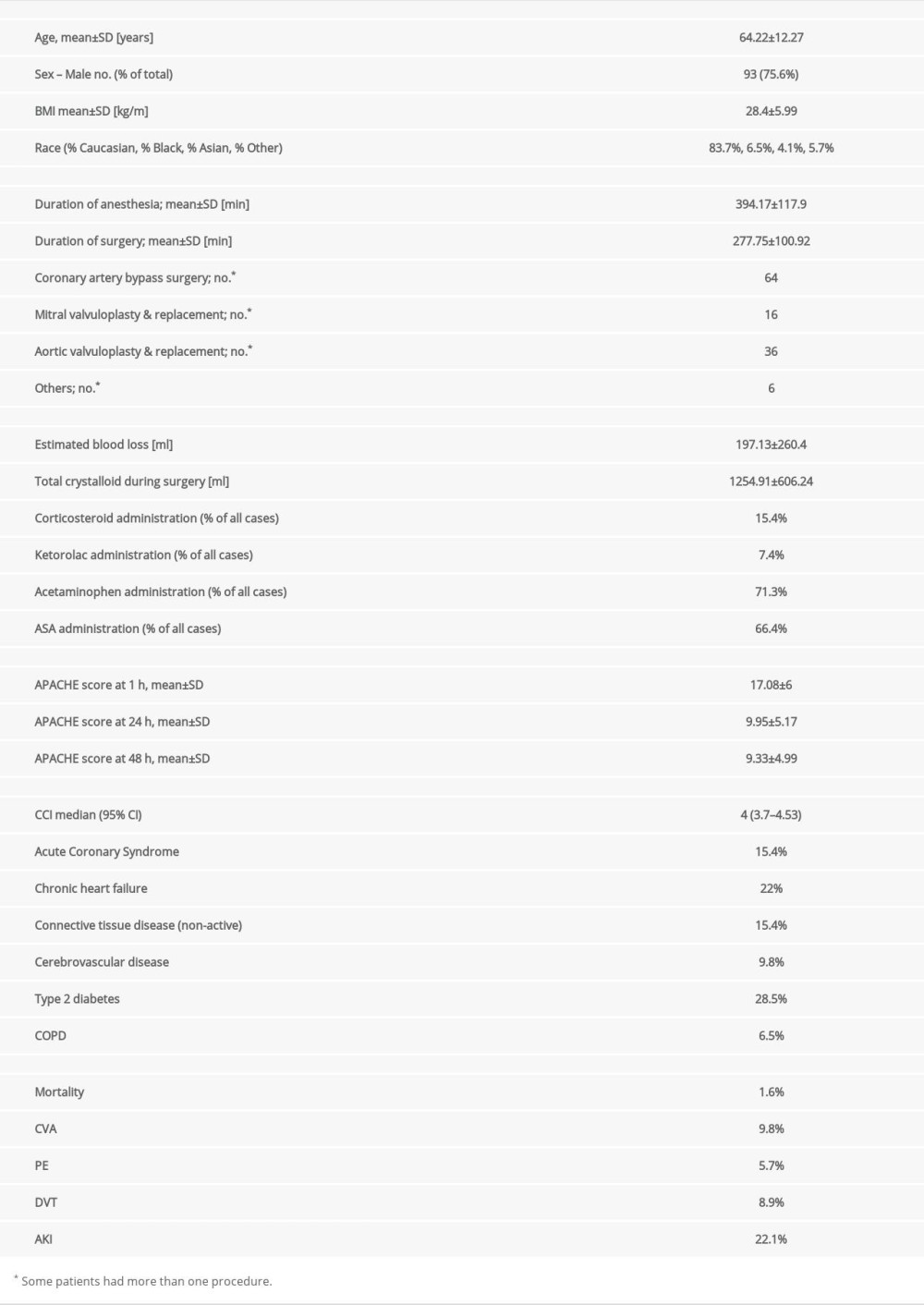
Sex or age over 60 years old did not affect serum leptin and FGF-21 levels at baseline. Asian patients had a significantly higher level of leptin (H[3;102]=9.23; P=0.029) at baseline, but only 5 patients of this race were enrolled in the study (Figure 1).
Patients with pre-existing diabetes had elevated serum leptin at tbaseline (leptinNoDM=Me[114.72;696] vs leptinDM=Me[320.04;802.4]; U[101]=2.205;
CHANGES IN LEPTIN AND FGF-21 AFTER CARDIAC SURGERY:
Sex, age, race, BMI, and collected history of pre-existing illnesses did not affect the leptin level at t24h, t7d, and t3m. Multiple regression analysis (including age, BMI, Charlson Comorbidity Index, procedure type, stay in ICU, and hospital duration of anesthesia and surgery, as well as APACHE at 1 and t24h after admission and baseline leptin demonstrated that the preoperative level of leptin accounted for 31% variation in the postoperative level at t24h (F[12;74]=5.44
We found that leptin levels increased significantly at t24h, then declined below baseline t7d after surgery, to normalize at t3m (U[60;3]=7.81; P=0.049) (Figure 2A). FGF-21 remained elevated at t24h and t7d (U[45;3]=21.38; P=0.0009) (Figure 2B). Patients undergoing mitral valve-related surgeries only had a transient increase in the acute postoperative period (P=0.033) (Figure 2C). Interestingly, patients with CABG had a somewhat steady level of leptin throughout all observation periods. Leptin and FGF-21 did not correlate significantly at t24h, t7d, and t3m.
Serum IL-6 levels (U[3;57]=62.16; P<0.00001) normalized at t3m after increases at t24h and t7d (Figure 2D). Serum CRP remained elevated even at t3m ([U3;26]=39.09; P<0.000001) (Figure 2E).
PERIOPERATIVE COURSE AND DYNAMICS OF LEPTIN AND FGF-21:
The increase in leptin and FGF-21 at t24h and t7d did not correlate with the duration of anesthesia, surgery, or time of the bypass. Estimated blood loss was weakly correlated with leptin level at t24h (r=0.2;
LEPTIN AND FGF-21 AND SHORT- AND LONG-TERM OUTCOME:
Leptin levels at t24h correlated weakly yet significantly with amyloid β1–42 but not with amyloid β1–40 at t3m (Figure 3). Central nervous system failure resulted in aberrations in FGF-21 at t24h (U[85;7]=2.42; P=0.01). Patients experiencing a stroke during surgery had lower baseline levels of FGF-21 (U[91;7]=2.24; P=0.023).
Leptin and FGF-21 serum levels did not correlate with APACHE scores at admission, t24h, and 48 h. Length of stay in the hospital or ICU did not correlate with leptin or FGF-21 levels at any time. Disposition at the discharge from the hospital indicated that FGF-21 at t24h (U[76;15]=1.97; P=0.049) and t7d (U[71;8]=2.06; P=0.038) was lower if the patient was transferred to the long-term facility versus to home discharge (Figure 4). Only 1 patient died in that group, rendering statistical analysis not feasible.
Discussion
This study encompassed a longitudinal sampling of patients who underwent heart surgery, with a follow-up at t3m. Our primary goal was to establish the relationship between serum leptin and FGF-21 longitudinal dynamics and postsurgical inflammatory and stress response, considering the function of these cytokines [9, 20–22]. We were particularly interested in the relationship of leptin and FGF-21 to presurgery BMI and pre-existing diabetes because of the role of leptin in long-term carbohydrate metabolism [2,4,5,9,21,23,24]. Pre-existing data suggested a link of leptin levels to susceptibility to pneumonia and sepsis as well as to long-term cognitive decline and cancer propagation [8,11,25–28]. FGF-21 modulates and augments several leptin functions, but no longitudinal studies of the serum hormone levels after cardiac surgery have been conducted to date [21,22].
Our study demonstrated that leptin levels peaked after around t7d and recovered to pre-baseline levels after t3m. This observation was similar to that of a prior study which detected normalization of the level by day 30 in patients undergoing cardiopulmonary bypass [5,7,13]. Our results showed that at t7d, leptin levels were lower, in contrast to another study that showed normalization of levels by day 4 [5,7,13]. Normalization by day 4 is likely part of a downward trend. We demonstrated that the type of surgery is one of the critical factors in determining the postsurgical dynamics of leptin. Patients undergoing arch repair had the highest baseline level but also had the steepest decline in leptin levels after surgery. Patients undergoing valve surgery exhibited a much more modest trend, while patients undergoing CABG had minimal leptin dynamics. The reason for these differences is unclear, but they have been related to the different etiologies leading to a particular surgery. The most common indication for arch surgery is aneurysmal changes, and leptin can accelerate the progression of this disease in animal models [29,30]. What is particularly interesting is that surgery of the arch dramatically reduces the level of leptin, suggesting removal of the source. This somewhat puzzling finding warrants further study to establish the causative effect. We expected that CABG would have the most pronounced level of leptin changes because of the role of leptin in sugar metabolism and progression of atherosclerosis via smoldering inflammation [5,8,9]. However, in the present study, CRP and IL-6 did not correlate with leptin levels, suggesting that leptin was an independent factor in postoperative inflammation. Also, the level of surgical insult measured via several perioperative factors demonstrated low correlations with leptin dynamics, suggesting that heart surgery by itself is a strong enough trigger for leptin release. This nonspecific role of leptin in modulating postsurgical response was noted in a study describing the pericardial fat release of leptin [10]. Interestingly, YKL-40 and leptin levels correlated, suggesting that ongoing tissue repair and leptin release may be interconnected, and that the serum leptin level may be potentially driven by epicardial fat [31,32]. Also, the pre-existing leptin levels were the most crucial variable in determining the postsurgical leptin response. A similar observation was made by Gu et al, in which preoperative leptin level was a risk factor for adverse effects after cardiac surgery [13]. That study was done in older Asian adults, suggesting that race may be a determining factor as it was described before [13]. This may suggest that there is a more complex interaction between long-term outcomes of surgery and race via leptin-driven mechanisms.
To date, the present study is the first long-term observation of the FGF-21 dynamic after cardiac surgery, except for one abstract that was published 10 years ago [33]. In our study, FGF-21 levels increased at t24h and fell below the pre-baseline levels at t3m. This profile was similar to that of IL-6 and CRP dynamics. Elevated FGF-21 levels correlated with central nervous system failure and the intake of benzodiazepines. Although central nervous system failure was defined alongside the clinical score, this observation was correlated with another study that showed an interaction between FGF-21 and the emergence of delirium or neurodegeneration [15,18,34]. The correlation between FGF-21 levels and the use of benzodiazepines may contribute to the difference in observed central nervous system failure. However, long-term decline was a predictor of unfavorable discharge, a finding well aligned with the critical role of FGF-21 in the metabolome, tissue healing, and neurodegeneration [34–37].
This study had several strengths. It had a large patient population, and we were able to study the presurgical state. Our sample was large and allowed us to make comparisons between baseline and later values. Because of the significant variability in baseline levels, the use of a longitudinal design was one of the most important advantages, specifically because hyperleptinemia is linked to persistent inflammation [22]. Also, we studied diverse adult patients in regards to sex, age, pre-existing medical conditions, and surgery type. We collected clinical data on the severity of surgery, perioperative management, and serum levels of inflammation markers (CRP, IL-6). Several pre-existing conditions important for leptin and FGF-21 levels were included in the data collection and analysis [15,22,24]. Finally, the methodology (enzyme-linked immunosorbent assay, multiplex) used to assess the serum level of the markers is very well established and robust, especially for measurements of IL-6, CRP, and FGF-21.
This was an exploratory study. Consequently, we could not conduct power analysis for FGF-21, in particular. In addition, our patient cohort was biased toward Whites and men; however, leptin levels are elevated in patients of Asian descent [13]. The significant limitation of the techniques used is relatively low sensitivity; however, only amyloid serum levels were close to the detection limits, potentially biasing the results. Furthermore, leptin levels are affected by glucocorticosteroids, circadian rhythm, and level of inflammation [9,38,39]. Our study did not account for these variables in an optimal way. Finally, FGF-21 levels may be affected by genetic variance [40].
Leptin and FGF are highly regulated by muscle and the liver [24]. Changes in muscle mass were not accounted for in this study. Liver function remained essentially nominal judging from liver injury markers; however, they can be a poor predictor of impaired synthetic function. There is a considerable amount of data linking leptin to immune-compromised immunity; however, in our sample, no patient experienced septic shock, and postoperative infectious complications were low [5,8,9]. Also, leptin biological activity is modulated by smoking and alcohol consumption, and we did not control for these factors. The correlations between leptin and FGF-21 and neuro-injury (amyloid) need to be quantified in more detail; however, FGF-21 seems to be generally protective in neuroinjury [19,34,35]. The abnormalities in FGF-21 and leptin may be a part of broader hyperglycemia and inflammation instead of being directly involved in the postsurgical neuroinflammation process. Next, studies should analyze the changes in leptin in conjunction with glucocorticosteroid metabolism, as leptin is dependent on steroids released during stress, in particular [9,24]. This is particularly important as exogenous steroids are used during some cardiac cases. There is a growing need to establish the role of leptin as the independent risk factor for postoperative complications in the context of pre-existing levels, race, and diabetes. Our pilot data demonstrated specific clinical correlates with neurodegeneration and cardiac remodeling markers; however, these findings must be validated with appropriate functional testing.
Conclusions
Leptin and FGF-21 evolve independently from the inflammatory response in the aftermath of cardiac surgery and correlate with cardiac remodeling and markers of neurodegeneration. Functional correlates of these correlations need to be established in the next study.
Figures
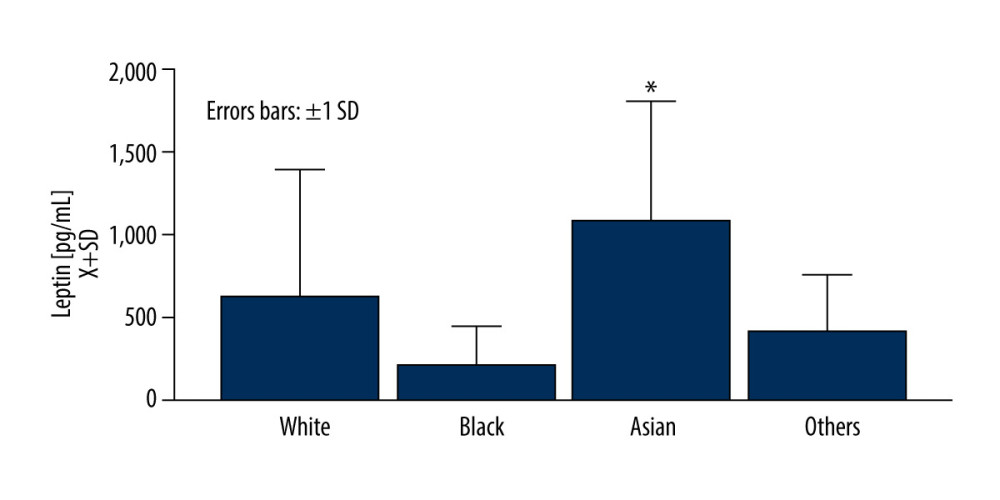 Figure 1. Asian race was related to an elevated level of leptin at the baselineFigures were generated using Prism by Graph Pad Dotmatics version 9. * Two-tailed significance less than 0.05.
Figure 1. Asian race was related to an elevated level of leptin at the baselineFigures were generated using Prism by Graph Pad Dotmatics version 9. * Two-tailed significance less than 0.05. 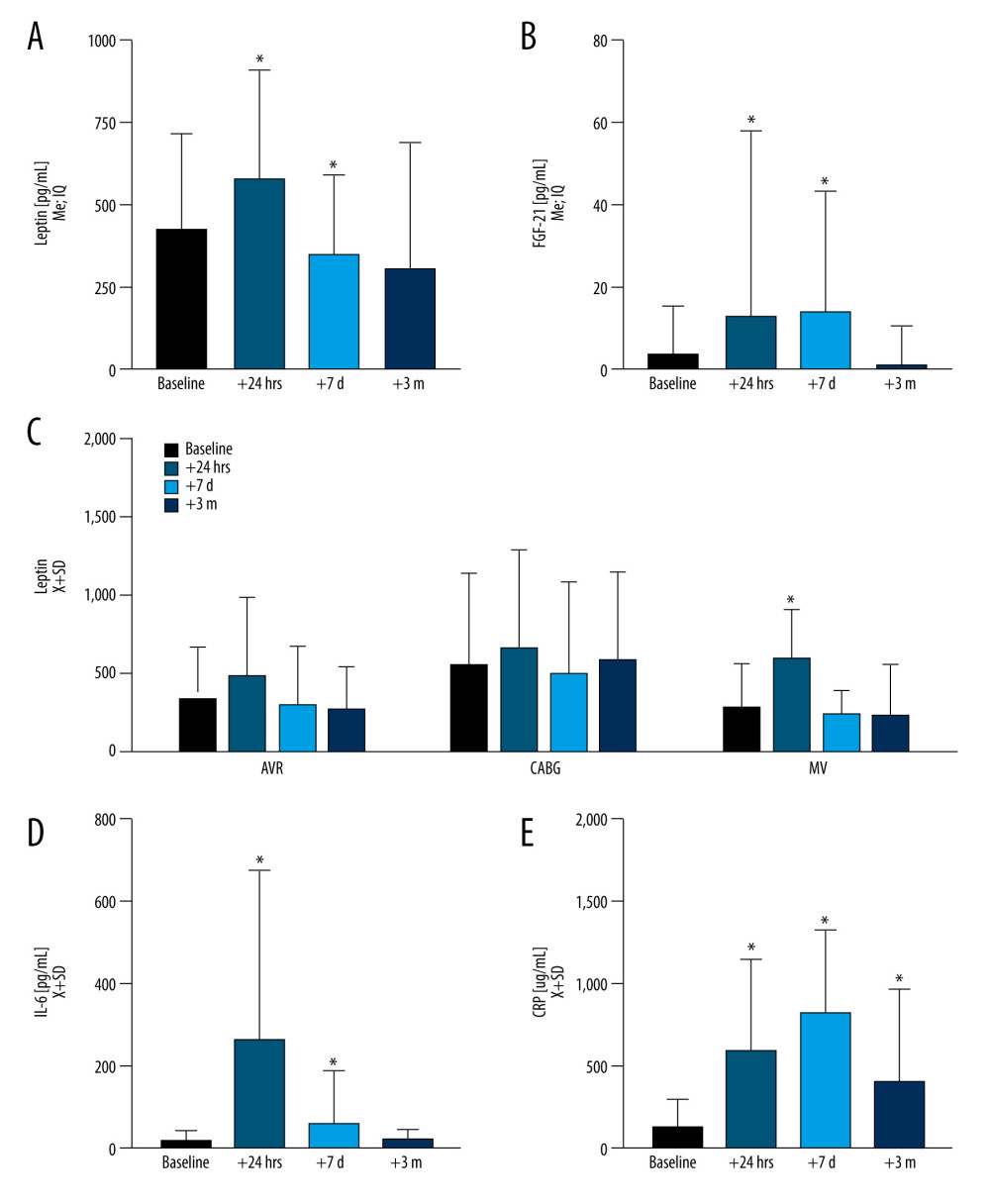 Figure 2. During the observation period, the dynamics of leptin and fibroblast growth factor 21 (FGF-21) as compared with inflammation markers, interleukin 6 (IL-6), C-reactive protein (CRP)(A) Leptin increased acutely at t24h (P<0.0001) to decline at t7d (P=0.043) while normalizing at t3m. (B) FGF-21 was elevated at both t24h (P<0.0001) and t7d (P<0.0001) and normalized at t3m. (C) Markers of acute inflammation, including IL-6 (D) were elevated at t24h (P<0.0001), while (E) CRP remained elevated across all of the time points (P<0.0001) as compared to baseline. Leptin levels slightly varied by surgery type at the baseline level and dynamics after surgery. Figures were generated using Prism by Graph Pad Dotmatics version 9. * Two-tailed significance less than 0.05 when data compared to baseline.
Figure 2. During the observation period, the dynamics of leptin and fibroblast growth factor 21 (FGF-21) as compared with inflammation markers, interleukin 6 (IL-6), C-reactive protein (CRP)(A) Leptin increased acutely at t24h (P<0.0001) to decline at t7d (P=0.043) while normalizing at t3m. (B) FGF-21 was elevated at both t24h (P<0.0001) and t7d (P<0.0001) and normalized at t3m. (C) Markers of acute inflammation, including IL-6 (D) were elevated at t24h (P<0.0001), while (E) CRP remained elevated across all of the time points (P<0.0001) as compared to baseline. Leptin levels slightly varied by surgery type at the baseline level and dynamics after surgery. Figures were generated using Prism by Graph Pad Dotmatics version 9. * Two-tailed significance less than 0.05 when data compared to baseline. 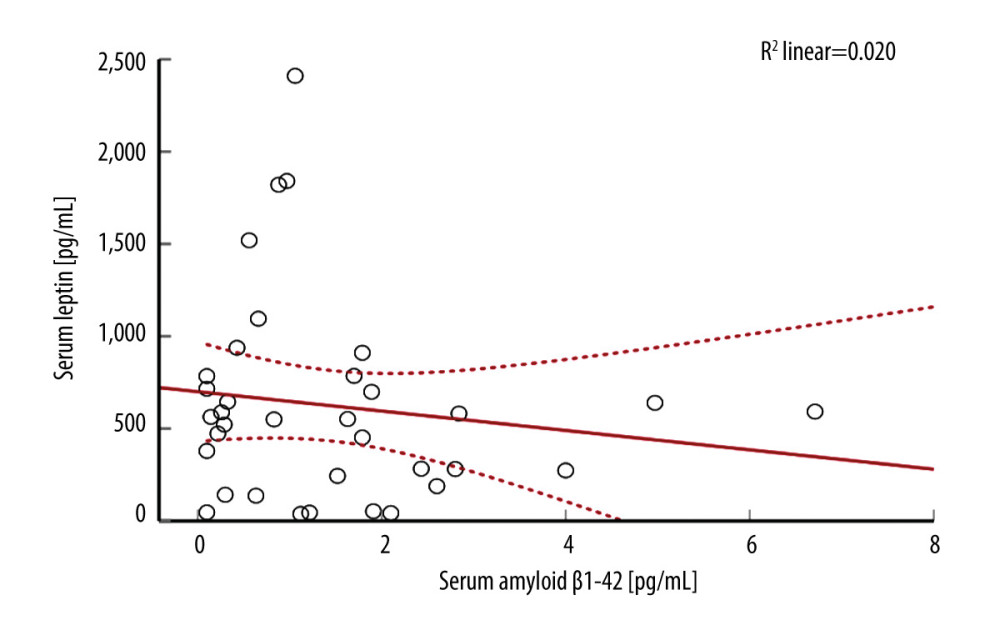 Figure 3. There was a weak correlation between serum amyloid β1–42 and leptin in the study populationFigures were generated using IBM SPSS Statistics version 27.
Figure 3. There was a weak correlation between serum amyloid β1–42 and leptin in the study populationFigures were generated using IBM SPSS Statistics version 27. 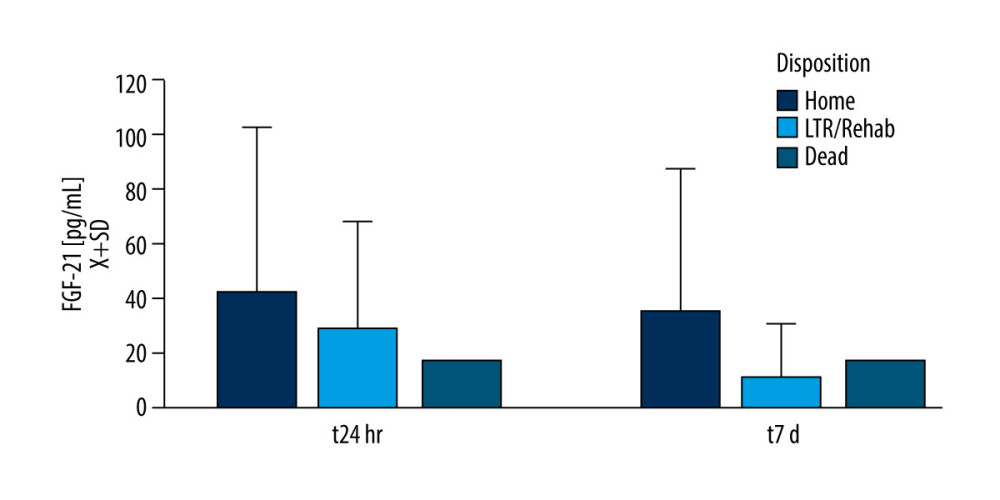 Figure 4. Fibroblast growth factor 21 (FGF-21) level and patient dispositionDischarge to the long-term rehabilitation facility instead of home was related to the lower serum level of FGF-21 at t24h and t7d. Only 1 patient died during the study duration. Figures were generated using Prism by Graph Pad Dotmatics version 9.
Figure 4. Fibroblast growth factor 21 (FGF-21) level and patient dispositionDischarge to the long-term rehabilitation facility instead of home was related to the lower serum level of FGF-21 at t24h and t7d. Only 1 patient died during the study duration. Figures were generated using Prism by Graph Pad Dotmatics version 9. References
1. Poetsch MS, Strano A, Guan K, Role of leptin in cardiovascular diseases: Front Endocrinol, 2020; 11; 354
2. Johnston KC, Bruno A, Pauls Q, Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: The SHINE Randomized Clinical Trial: JAMA, 2019; 322(4); 326-35
3. Wilcox ME, Girard TD, Hough CL, Delirium and long term cognition in critically ill patients: BMJ, 2021; 373; n1007
4. Perrotti A, Ecarnot F, Monaco F, Quality of life 10 years after cardiac surgery in adults: A long-term follow-up study: Health Qual Life Outcomes, 2019; 17(1); 88
5. Modan-Moses D, Kanety H, Dagan O, Leptin and the post-operative inflammatory response. More insights into the correlation with the clinical course and glucocorticoid administration: J Endocrinol Invest, 2010; 33(10); 701-6
6. Caldwell A, Morick JN, Jentsch AM, Impact of insulin on the intestinal microcirculation in a model of sepsis-related hyperglycemia: Microvasc Res, 2018; 119; 117-28
7. Lena Gunningberg ML, Serum leptin is decreased thirty days after surgery: Journal of Diabetes & Metabolism, 2014; 5(12); 2155-6156.1000465
8. Lord GM, Matarese G, Howard JK, Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression: Nature, 1998; 394; 897-901
9. Heiman ML, Ahima RS, Craft LS, Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress: Endocrinology, 1997; 138(9); 3859-63
10. Mach L, Bedanova H, Soucek M, Impact of cardiopulmonary bypass surgery on cytokines in epicardial adipose tissue: Comparison with subcutaneous fat: Perfusion, 2017; 32(4); 279-84
11. Caldefie-Chezet F, Poulin A, Leptin: A potential regulator of polymorphonuclear neutrophil bactericidal action?: J Leukoc Biol, 2001; 69(3); 414-18
12. Arnalich F, López J, Codoceo R, Relationship of plasma leptin to plasma cytokines and human survivalin sepsis and septic shock: J Infect Dis, 1999; 180(3); 908-11
13. Gu Z, Sun C, Xiang D, Postoperative adverse cardiovascular events associated with leptin and adverse age after elective major non-cardiac surgery: An Asian Single-Center Study: Med Sci Monit, 2018; 24; 2119-25
14. Sun M, Jin L, Bai Y, Fibroblast growth factor 21 protects against pathological cardiac remodeling by modulating galectin-3 expression: J Cell Biochem, 2019; 120(12); 19529-40
15. McKay TB, Rhee J, Colon K, Preliminary study of serum biomarkers associated with delirium after major cardiac surgery: J Cardiothorac Vasc Anesth, 2022; 36(1); 118-24
16. Cleves MA, Sanchez N, Draheim M, Evaluation of two competing methods for calculating Charlson’s comorbidity index when analyzing short-term mortality using administrative data: J Clin Epidemiol, 1997; 50(8); 903-8
17. Jiang L, Feng B, Gao D, Zhang Y, Plasma concentrations of copeptin, C-reactive protein and procalcitonin are positively correlated with APACHE II scores in patients with sepsis: J Int Med Res, 2015; 43(2); 188-95
18. Peres Bota D, Melot C, Lopes Ferreira F, The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction: Intensive Care Med, 2002; 28(11); 1619-24
19. Lue L-F, Guerra A, Walker DG, Amyloid beta and tau as Alzheimer’s disease blood biomarkers: Promise from new technologies: Neurol Ther, 2017; 6(Suppl 1); 25-36
20. Ubags ND, Stapleton RD, Vernooy JH, Hyperleptinemia is associated with impaired pulmonary host defense: JCI Insight, 2016; 1(8); e82101
21. Zhang Y, Xie Y, Berglund ED, The starvation hormone, fibroblast growth factor-21, extends lifespan in mice: eLife, 2012; 1; e00065
22. Polyakova EA, Mikhaylov EN, Galagudza MM, Shlyakhto EV, Hyperleptinemia results in systemic inflammation and the exacerbation of ischemia-reperfusion myocardial injury: Heliyon, 2021; 7(11); e08491
23. Wernly B, Lichtenauer M, Franz M, Differential impact of hyperglycemia in critically ill patients: Significance in acute myocardial infarction but not in sepsis?: Int J Mol Sci, 2016; 17(9); 1586
24. Katsiki N, Mikhailidis DP, Banach M, Leptin, cardiovascular diseases and type 2 diabetes mellitus: Acta Pharmacol Sin, 2018; 39(7); 1176-88
25. Lieb W, Beiser AS, Vasan RS, Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging: JAMA, 2009; 302(23); 2565-72
26. Westwood AJ, Beiser A, Decarli C, Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy: Neurology, 2014; 82(18); 1613-19
27. Kang SY, Kim YJ, Jang W, Body mass index trajectories and the risk for Alzheimer’s disease among older adults: Sci Rep, 2021; 11(1); 3087
28. Perrier S, Caldefie-Chézet F, Vasson MP, IL-1 family in breast cancer: Potential interplay with leptin and other adipocytokines: FEBS Lett, 2009; 583(2); 259-65
29. Ben-Zvi D, Savion N, Kolodgie F, Local application of leptin antagonist attenuates angiotensin ii-induced ascending aortic aneurysm and cardiac remodeling: J Am Heart Assoc, 2016; 5(5); e003474
30. Tao M, Yu P, Nguyen BT, Locally applied leptin induces regional aortic wall degeneration preceding aneurysm formation in apolipoprotein E – deficient mice: Arterioscler Thromb Vasc Biol, 2013; 33(2); 311-20
31. Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG, Elevated plasma YKL-40, lipids and lipoproteins, and ischemic vascular disease in the general population: Stroke, 2015; 46(2); 329-35
32. Rathcke CN, Vestergaard H, YKL-40 – an emerging biomarker in cardiovascular disease and diabetes: Cardiovasc Diabetol, 2009; 8(1); 61
33. Drapalova J, Kotulak T, Kopecky P, Changes in serum concentrations and tissue expression of fibroblast growth factor-21 during and after elective major cardiac surgery: Endocrine Abstracts, 2010; 22; P159
34. Chen S, Chen ST, Sun Y, Fibroblast growth factor 21 ameliorates neurodegeneration in rat and cellular models of Alzheimer’s disease: Redox Biol, 2019; 22; 101133
35. Pekkarinen PT, Skrifvars MB, Lievonen V, Serum fibroblast growth factor 21 levels after out of hospital cardiac arrest are associated with neurological outcome: Sci Rep, 2021; 11(1); 690
36. Wu L, Qian L, Zhang L, Fibroblast growth factor 21 is related to atherosclerosis independent of nonalcoholic fatty liver disease and predicts atherosclerotic cardiovascular events: J Am Heart Assoc, 2020; 9(11); e015226
37. Gan F, Huang J, Dai TLM, Liu J, Serum level of fibroblast growth factor 21 predicts long-term prognosis in patients with both diabetes mellitus and coronary artery calcification: Ann Palliat Med, 2020; 9(2); 368-74
38. Licinio J, Mantzoros C, Negrão AB, Human leptin levels are pulsatile and inversely related to pituitary-adrenal function: Nat Med, 1997; 3(5); 575-79
39. Hoda MR, El-Achkar H, Schmitz E, Systemic stress hormone response in patients undergoing open heart surgery with or without cardiopulmonary bypass: Ann Thorac Surg, 2006; 82(6); 2179-86
40. Shabana , Hasnain S, Leptin promoter variant G2548A is associated with serum leptin and HDL-C levels in a case control observational study in association with obesity in a Pakistani cohort: J Biosci, 2016; 41(2); 251-55
Figures
 Figure 1. Asian race was related to an elevated level of leptin at the baselineFigures were generated using Prism by Graph Pad Dotmatics version 9. * Two-tailed significance less than 0.05.
Figure 1. Asian race was related to an elevated level of leptin at the baselineFigures were generated using Prism by Graph Pad Dotmatics version 9. * Two-tailed significance less than 0.05. Figure 2. During the observation period, the dynamics of leptin and fibroblast growth factor 21 (FGF-21) as compared with inflammation markers, interleukin 6 (IL-6), C-reactive protein (CRP)(A) Leptin increased acutely at t24h (P<0.0001) to decline at t7d (P=0.043) while normalizing at t3m. (B) FGF-21 was elevated at both t24h (P<0.0001) and t7d (P<0.0001) and normalized at t3m. (C) Markers of acute inflammation, including IL-6 (D) were elevated at t24h (P<0.0001), while (E) CRP remained elevated across all of the time points (P<0.0001) as compared to baseline. Leptin levels slightly varied by surgery type at the baseline level and dynamics after surgery. Figures were generated using Prism by Graph Pad Dotmatics version 9. * Two-tailed significance less than 0.05 when data compared to baseline.
Figure 2. During the observation period, the dynamics of leptin and fibroblast growth factor 21 (FGF-21) as compared with inflammation markers, interleukin 6 (IL-6), C-reactive protein (CRP)(A) Leptin increased acutely at t24h (P<0.0001) to decline at t7d (P=0.043) while normalizing at t3m. (B) FGF-21 was elevated at both t24h (P<0.0001) and t7d (P<0.0001) and normalized at t3m. (C) Markers of acute inflammation, including IL-6 (D) were elevated at t24h (P<0.0001), while (E) CRP remained elevated across all of the time points (P<0.0001) as compared to baseline. Leptin levels slightly varied by surgery type at the baseline level and dynamics after surgery. Figures were generated using Prism by Graph Pad Dotmatics version 9. * Two-tailed significance less than 0.05 when data compared to baseline. Figure 3. There was a weak correlation between serum amyloid β1–42 and leptin in the study populationFigures were generated using IBM SPSS Statistics version 27.
Figure 3. There was a weak correlation between serum amyloid β1–42 and leptin in the study populationFigures were generated using IBM SPSS Statistics version 27. Figure 4. Fibroblast growth factor 21 (FGF-21) level and patient dispositionDischarge to the long-term rehabilitation facility instead of home was related to the lower serum level of FGF-21 at t24h and t7d. Only 1 patient died during the study duration. Figures were generated using Prism by Graph Pad Dotmatics version 9.
Figure 4. Fibroblast growth factor 21 (FGF-21) level and patient dispositionDischarge to the long-term rehabilitation facility instead of home was related to the lower serum level of FGF-21 at t24h and t7d. Only 1 patient died during the study duration. Figures were generated using Prism by Graph Pad Dotmatics version 9. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952