05 October 2022: Clinical Research
Performance of Xpert MTB/RIF for Diagnosis of Tuberculosis in HIV-Infected People in China: A Retrospective, Single-Center Study
Guiju Gao1ACDEFG*, Chunyu Zhu2BCDEF, Yingchu Liu3BCDEF, Siyuan YangDOI: 10.12659/MSM.937264
Med Sci Monit 2022; 28:e937264
Abstract
BACKGROUND: There are few studies of GeneXpert MTB/RIF (hereafter referred to as Xpert) detection technology in HIV-infected people in China. Therefore, this study aimed to evaluate the value of Xpert in HIV/TB co-infected patients and to provide reference and guidance for the diagnosis of TB in HIV-infected populations.
MATERIAL AND METHODS: This study reviewed medical records of human immunodeficiency virus (HIV) patients hospitalized at the Infection Center of Beijing Ditan Hospital affiliated to Capital Medical University from January 2018 to May 2020, and patients diagnosed with pulmonary and extrapulmonary tuberculosis were screened as study subjects. Sensitivity and specificity of Xpert were analyzed using ROC curves.
RESULTS: Of the 413 HIV patients, 177 patients met the entry criteria, of which the diagnosis was active pulmonary tuberculosis (PTB): 145 and extrapulmonary tuberculosis (EPTB): 32. The sensitivity of Xpert for PTB and EPTB was 82.0% and 100%, higher than that of acid-fast bacilli (AFB) (61.0% and 58.3%), and slightly lower than that of T-SPOT.TB (91.0% and 100%); the specificity was 83.7% and 93.5%, higher than that of AFB (72.6%, 87.1%) and T-SPOT.TB (16.6%, 21.2%). The sensitivity of Xpert was 100% in bronchoalveolar lavage fluid (BALF) and 80.0% in sputum; in patients with CD4⁺ <200 cells/mm³, the sensitivity of Xpert was 90.0% and specificity was 84.8%, higher than that of AFB (60.0%, 75.5%) and T-SPOT.TB (90.0%, 21.5%).
CONCLUSIONS: Xpert has a high accuracy in HIV/TB co-infected patients, and Xpert still shows a high sensitivity and specificity even in HIV patients with CD4⁺ <200 cells/mm³. Xpert is recommended for the diagnosis of tuberculosis in HIV-infected patients.
Keywords: AIDS-Related Opportunistic Infections, Microbial Sensitivity Tests, Mycobacterium tuberculosis, HIV Infections, Humans, Rifampin, Sensitivity and Specificity, Sputum, Tuberculosis, Tuberculosis, Pulmonary
Background
Currently, Acquired Immune Deficiency Syndrome (AIDS) and tuberculosis (TB) remain major public health problems. According to the World Health Organization’s 2020 Global Tuberculosis Report, there will be approximately 820 000 people with HIV/TB co-infection globally (8.2% of all TB patients) in 2020, of which 214 000 will die from HIV/TB co-infection [1]. Considering that HIV populations are at much higher risk of
Traditionally,
Material and Methods
STUDY DESIGN AND PATIENTS:
This study was conducted at Beijing Ditan Hospital affiliated to Capital Medical University, Beijing, China, which is a specialist hospital for the infectious diseases. From January 2018 to May 2020, a total of 413 HIV patients were included in this study; all patients were uniformly tested and patient medical records were available. We established the following screening criteria according to the World Health Organization (WHO) guidelines [8] and the guidelines of the National Health and Wellness Commission of the People’s Republic of China [9]: (1) age >18 years old and HIV-1 antibody-positive; (2) exclusion of HIV patients in the acute infection phase or patients with combined malignancy; (3) PTB patients with MTB culture-positive, or AFB smear-positive and imaging findings consistent with the characteristics of active pulmonary tuberculosis; (4) EPTB patients with definite tuberculosis involving organs other than the lungs, MTB from non-pulmonary origin tissue isolated, or with strong clinical evidence consistent with active EPTB histology. Ultimately, 236 patients were excluded from the analysis (139 non-TB patients, 42 missing culture results, 20 missing Xpert results, 29 missing AFB results, and 6 missing specimens). Therefore, 177 patients were included in the final analysis: PTB (145) and EPTB (32) (Figure 1).
TEST METHOD:
With the MTB culture method, collected specimens were placed in centrifuge tubes, treated with N-acetylcysteine and sodium hydroxide, shaken for 20 s to fully mix, and let stand at room temperature for 15 min, then centrifuged at 3000 r/min in the vessel and resuspended in 1.5 ml phosphate buffer. The BACTEC MGIT 960 system (BD microbial system) was used to inoculate the prepared sample into the solid medium.
With AFB smear microscopy, according to the operation requirements of AFB smear microscopy, 300 different fields are randomly selected for testing. If acid-fast bacilli were found, it was positive, otherwise it was negative.
With the Interferon-gamma release assay (by T-SPOT.TB), the heparinized blood of patients was collected and used for the T-SPOT®.TB test (Oxford Immunotec, Oxford, UK) in accordance with the manufacturer’s instructions. When the negative and positive quality controls were under control, the results were considered positive if either panel A or panel B had ≥6 spots.
With the Xpert test, Xpert MTB/RIF reagent was added to the specimen at a ratio of 2: 1. If the sample volume was less than 1 ml, we added reagents to the sample at a ratio of 3: 1 to meet the volume requirements, and centrifuged for 15 min. The sealed specimen was vigorously stirred at room temperature for 15 min. Then, we transferred 2 ml of sample to the kit and inserted it into the test platform. Xpert automatically performs DNA extraction, nucleic acid amplification, and specific nucleic acid detection of
STATISTICAL ANALYSIS:
For continuous variables, the data are expressed as median and interquartile spacing. The chi-square test and rank sum test were used to compare culture-positive and culture-negative groups. With sputum
ETHICAL APPROVAL:
The study was approved by the Ethics Review Committee of Beijing Ditan Hospital (approval number: 201704002).
Results
PATIENT CHARACTERISTICS:
In this study, 177 patients were included. The median age was 37 (20–83) years, there were 66 (37.2%) patients with a history of tuberculosis; 17(5.7%) were female and 160 (94.3%) were male, with a median CD4 count of 103 (9–886). The main clinical symptoms were fever (72.4%), cough (38.7%), sputum (21.4%), night sweats (1.1%), and weight loss (3.3%); the imaging manifestations were nodules (80.6%), patchy shadow (34.6%), and pleural effusion (33.6%) (Table 1).
PERFORMANCE OF XPERT, AFB, T-SPOT.TB IN PTB, EPTB, AND TOTAL CASES:
The positivity rates of Xpert, AFB, and T-SPOT.TB were 42.3% (75/177), 32.7% (58/177), and 72.8% (129/177), respectively. Compared with the criterion standard, the diagnostic performance of AFB (AUC=0.713, sensitivity 58.3%, specificity 76.3%) was significantly better than that of T-SPOT.TB (AUC=0.564, sensitivity 91.6%, specificity 21.2%) (P<0.001). Xpert had the best diagnostic performance (AUC=0.798, sensitivity 83.3%, specificity 84.2%), which was significantly higher than the other 2 methods (P<0.001) (Figure 2, Table 2).
ACCURACY OF XPERT IN DIFFERENT SAMPLES OF PTB AND EPTB:
In pulmonary samples, BALF had higher sensitivity (100%,95% CI: 19.7–100) and specificity (81.2%,95% CI: 69.1–89.5) than sputum. Among extrapulmonary samples, CSF specificity was the highest (95.0%, 95% CI: 73.1–99.7), and urine specificity was the lowest (50.0%, 95% CI: 2.6–97.3).
:
For patients with CD4+<200 cells/mm3, the sensitivity of Xpert and T-SPOT.TB were both 90.0% (95% CI: 54.1–99.4%), which was significantly higher than that of AFB: 60.0% (95% CI: 27.3–86.3%) (
Discussion
Xpert is a new technology for rapid detection of tuberculosis and drug resistance recommended globally by the WHO, and with advances in molecular biology, this test is increasingly being used in China. This study confirmed the superior diagnostic performance of Xpert (AUC=0.798, sensitivity: 83.3%, specificity: 84.2%), which was significantly higher than that of AFB and T-SPOT.TB (
According to our findings, 37.2% of HIV/TB patients had a history of tuberculosis, which may indicate that a history of previous tuberculosis is informative for the diagnosis of tuberculosis in the HIV-infected population [11]. However, in patients with previous TB, antigen-specific memory T cells may be present in the body for a long time, which can lead to false-positive results [5,11]. Therefore, the diagnosis of TB is more challenging in HIV-positive than in HIV-negative patients.
Opinions differ in several Xpert studies on different sample types (eg, sputum, BALF, CSF, pleural fluid, urine, blood, abscess aspirate) [4,10,12], but its accuracy is important for MTB detection. Previous studies have shown that Xpert has sufficient accuracy in diagnosing PTB, but lacks good accuracy in diagnosing EPTB [13,14]. In our study, Xpert examined 32 extrapulmonary specimens (20 CSF, 1 ascites specimen, 2 urine specimens, and 3 pleural fluid specimens) (see Table 3). There was no significant difference between extrapulmonary specimen types and Xpert assay performance. In particular, there were no positive test results for CSF samples, which may be due to the paucibacillary nature of the extrapulmonary specimens, which is roughly the same as in another study [15]. Moreover, invasive specimen collection for microbiological diagnosis by biopsy or fine-needle aspiration for EPTB patients, which makes bacteriological confirmation more difficult [16].
Nontuberculous mycobacteria (NTM) such as
We compared the sensitivity and specificity of AFB, Xpert and T-SOPT.TB in patients with CD4+ <200 cells/mm3. The results showed that Xpert had higher sensitivity and specificity than AFB, but the specificity of Xpert decreased compared to the overall data, which is the same as the results of another study, in which the accuracy of Xpert in diagnosing PTB was decreased in patients with CD4+ <200 cells/mm3, but the difference was not statistically significant [11] (see Table 4).
In addition, the positive rate of rifampicin resistance in this study was 9.6% (17/177) (for information only), which was higher than the average level of multidrug resistance in China in 2012 (8.3%) [19], and lower than in a 2018 study in a chest hospital in Beijing, China (61.9%, 13/21) [20]. This suggests that the burden of multidrug-resistant tuberculosis (MDR-TB) in China is severely underestimated and that universal access to Xpert testing technology is imminent. Also, HIV-positive patients are at higher risk of developing MDR-TB than other populations, and HIV-positive patients with MDR-TB have poorer treatment outcomes than HIV-negative patients with MDR-TB [21,22]. Therefore, it is necessary to monitor the occurrence of tuberculosis and rifampicin resistance in HIV-positive people, and to maintain good tuberculosis prevention and treatment practices [23].
Our study has some limitations. This was a single-center retrospective study, which may limit the generalizability of the findings. The study is limited by the small sample size of EPTB. The use of Xpert for the diagnosis of EPTB needs to be further investigated by expanding the sample size and optimizing the sample handling process for EPTB to draw more accurate conclusions. There will be uncertainty in the results of Xpert MTB/RIF test for oligobacterium samples, and when HIV patients are infected with tuberculosis, the sensitivity of Xpert diagnosis will be reduced. Therefore, the subsequent development of Xpert Ultra aims to improve the sensitivity of tuberculosis detection, especially in AFB-negative patients and HIV-positive patients [24]. In addition, Xpert MTB/RIF may have false-positive results of rifampicin resistance when initially testing rifampicin-resistant patients [25]. Finally, we did not include HIV-negative patients. However, some studies reported that the sensitivity of Xpert was higher in HIV-negative people than in HIV- positive people [12].
Conclusions
In conclusion, our study showed that the specificity of Xpert was higher than that of AFB and T-SPOT.TB, and even in HIV patients with CD4+<200 cells/mm3, Xpert still showed high accuracy. Our results support the use of Xpert as a diagnostic method for TB in HIV patients, and Xpert has outstanding advantages in detecting rifampicin resistance.
Figures
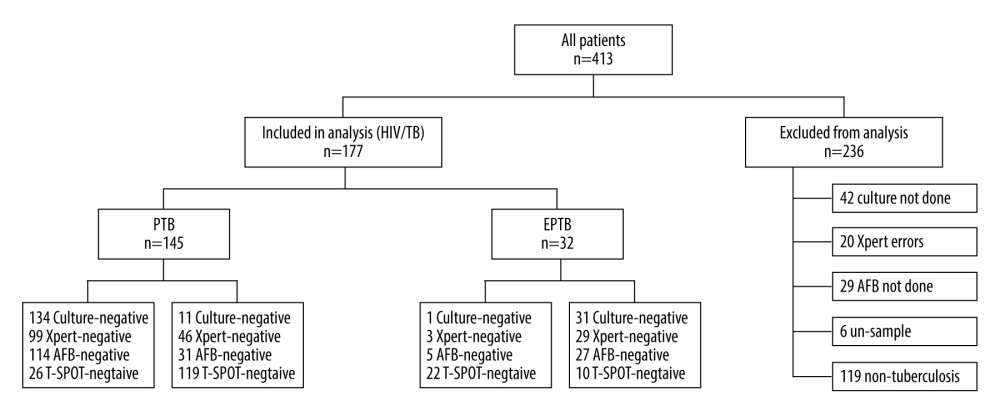 Figure 1. Of the 413 HIV patients, 177 patients met the inclusion criteria, including those diagnosed with active PTB (145), and EPTB (32); 236 patients were excluded from the analysis. (PowerPoint, 2016 version, Microsoft).
Figure 1. Of the 413 HIV patients, 177 patients met the inclusion criteria, including those diagnosed with active PTB (145), and EPTB (32); 236 patients were excluded from the analysis. (PowerPoint, 2016 version, Microsoft). 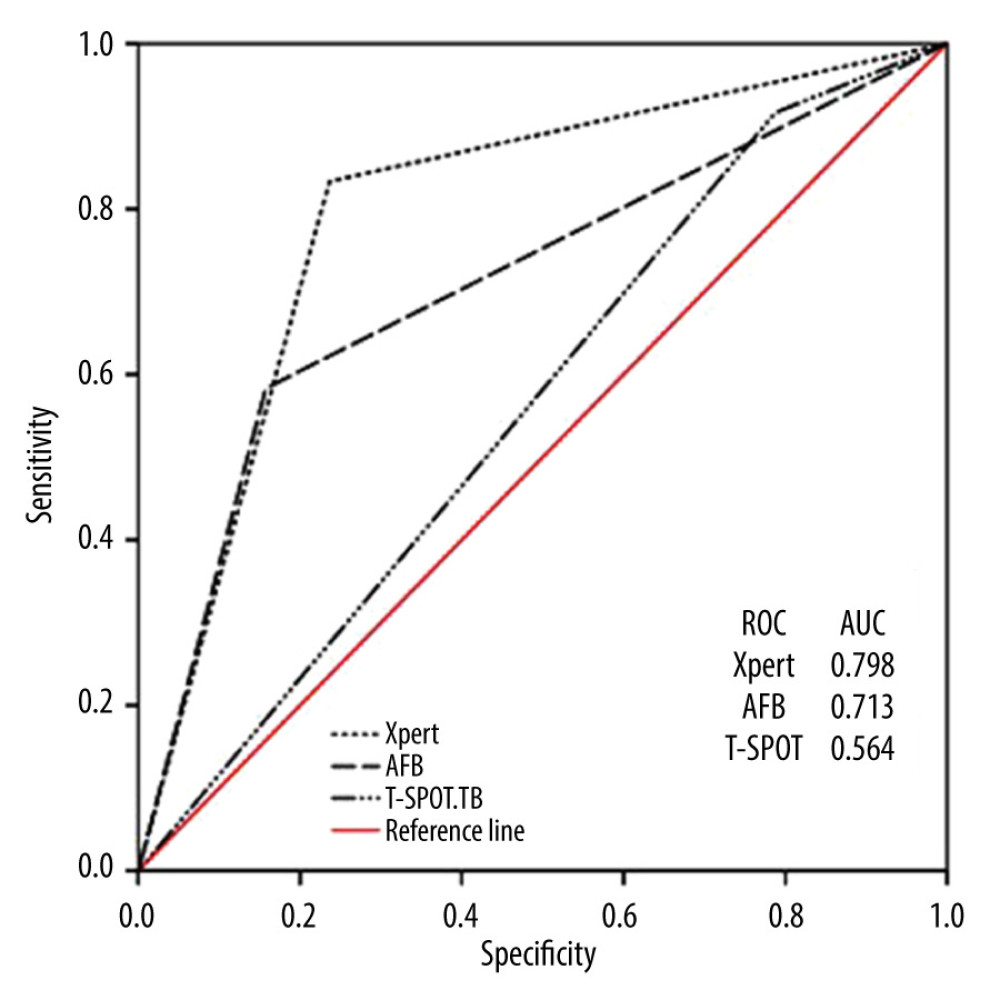 Figure 2. Receiver operating curves (ROC) for Xpert, Acid-fast bacilli (AFB) and T-SPOT.TB to differentiate tuberculosis (TB) and non-TB cases. AUC, area under the ROC. (SPSS, 21 version, Microsoft).
Figure 2. Receiver operating curves (ROC) for Xpert, Acid-fast bacilli (AFB) and T-SPOT.TB to differentiate tuberculosis (TB) and non-TB cases. AUC, area under the ROC. (SPSS, 21 version, Microsoft). Tables
Table 1. Demographic characteristics of enrolled patients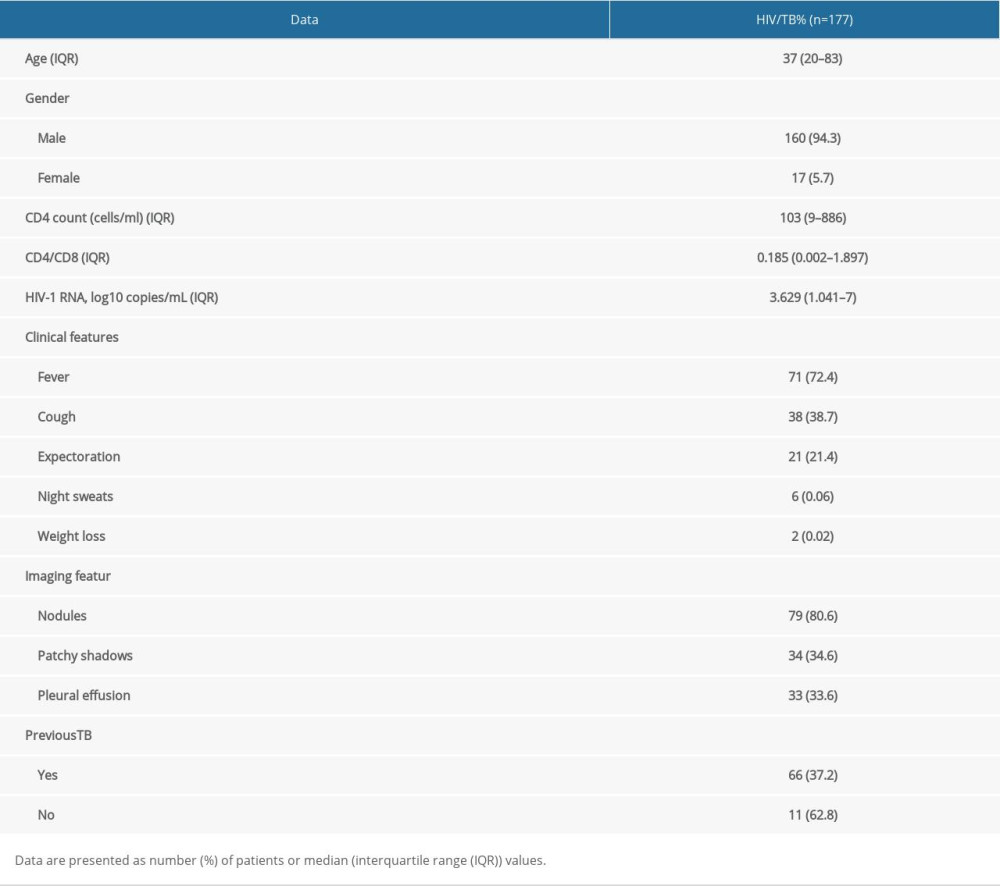 Table 2. Performance of Xpert, AFB and T-SPOT in detecting PTB, EPTB and total cases.
Table 2. Performance of Xpert, AFB and T-SPOT in detecting PTB, EPTB and total cases.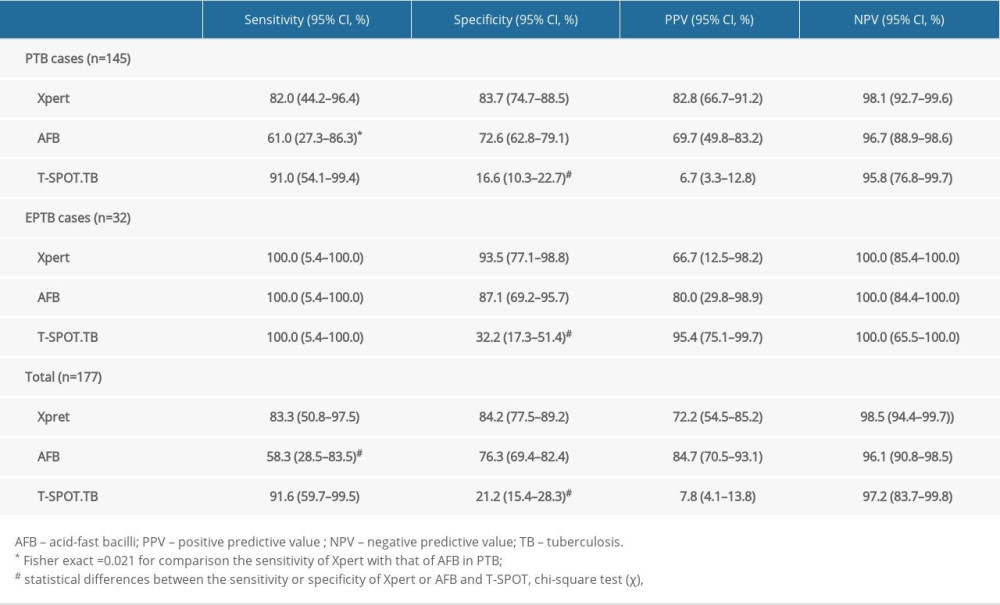 Table 3. Sensitivity and specificity of Xpert in different specimens.
Table 3. Sensitivity and specificity of Xpert in different specimens.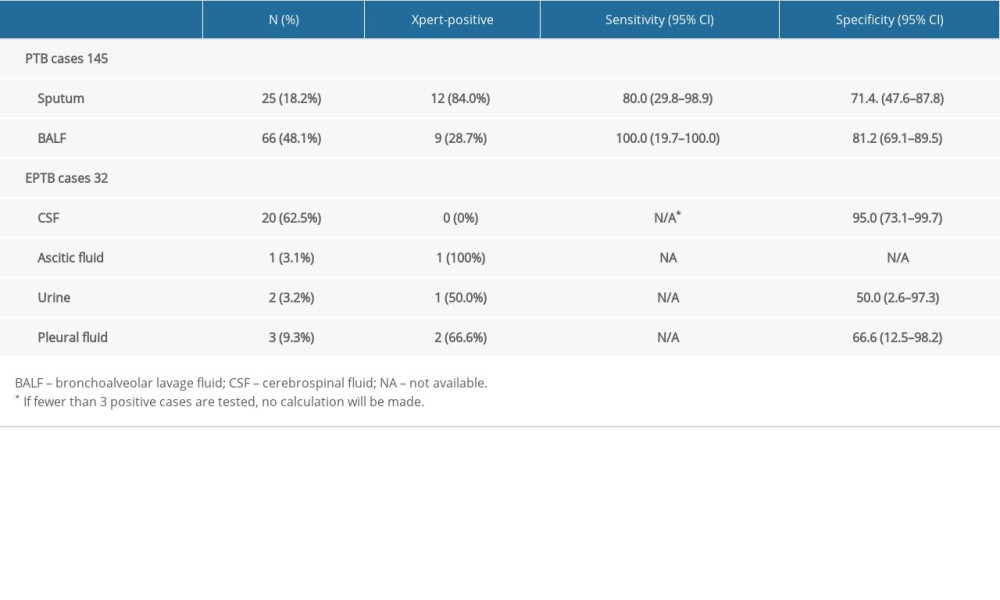 Table 4. Comparison of the performance of Xpert, AFB and T-SPOT.TB in the diagnosis of pulmonary tuberculosis when CD4+ <200 cells/mm3.
Table 4. Comparison of the performance of Xpert, AFB and T-SPOT.TB in the diagnosis of pulmonary tuberculosis when CD4+ <200 cells/mm3.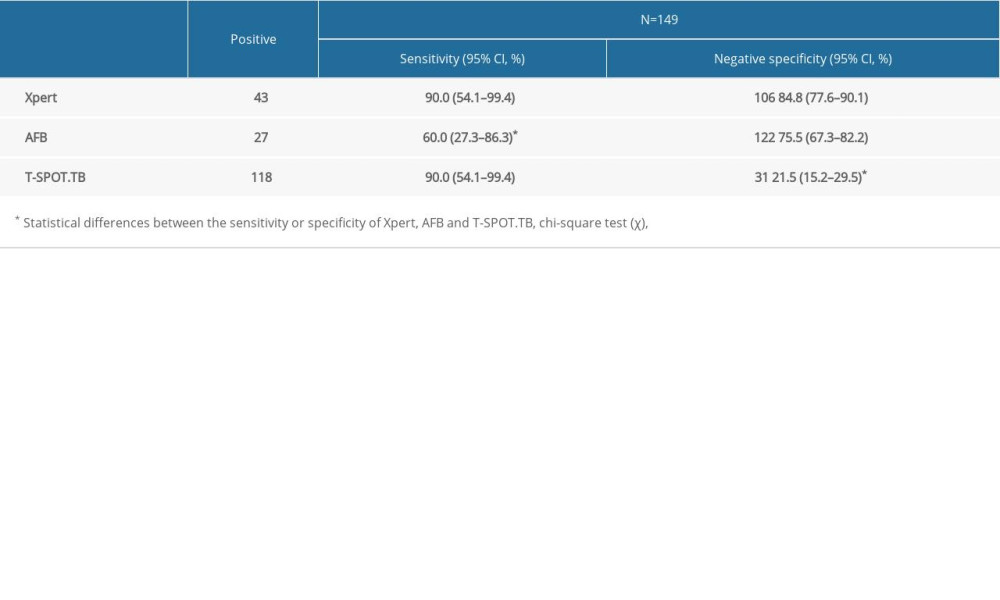
References
1. World Health Organization: Global tuberculosis report, 2020
2. World Health Organization: Global tuberculosis report, 2017
3. Deshwal H, Avasarala SK, Ghosh S, Mehta AC, Forbearance with bronchoscopy: A review of gratuitous indications: Chest, 2019; 155(4); 834-47
4. Bahr NC, Nuwagira E, Evans EE, Diagnostic accuracy of Xpert MTB/RIF ultra for tuberculous meningitis in HIV-infected adults: A prospective cohort study: Lancet Infect Dis, 2018; 18(1); 68-75
5. Pan L, Jia H, Liu F, Risk factors for false-negative T-SPOT.TB assay results in patients with pulmonary and extra-pulmonary TB: J Infect, 2015; 70(4); 367-80
6. World Health Organization: Global tuberculosis report, 2010
7. World Health Organization: Global tuberculosis report, 2014
8. World Health Organization: The treatment of tuberculosis: guidelines Available: https://www.who.int/tb/publications/tb_treatmentguidelines/en/
9. National Health and Family Planning Commission of the People’s Republic of China: Diagnosis for pulmonary tuberculosis (WS 288-2017), 2017
10. Maynard-Smith L, Larke N, Peters JA, Lawn SD, Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: A systematic review: BMC Infect Dis, 2014; 14; 709
11. Zhang P, Liu H, Wang H, Performance of Xpert MTB/RIF Ultra for the diagnosis of pulmonary tuberculosis using bronchoalveolar lavage samples in people living with HIV/AIDS (PLWHA) in China: A prospective study: HIV AIDS (Auckl), 2021; 13; 905-16
12. Luetkemeyer AF, Firnhaber C, Kendall MA, Evaluation of Xpert MTB/RIF versus AFB Smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings: Clin Infect Dis, 2016; 62(9); 1081-88
13. Dorman SE, Schumacher SG, Alland D: Lancet Infect Dis, 2018; 18(1); 76-84
14. Denkinger CM, Schumacher SG, Boehme CC, Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: A systematic review and meta-analysis: Eur Respir J, 2014; 44(2); 435-46
15. Zürcher K, Ballif M, Kiertiburanakul S, Diagnosis and clinical outcomes of extrapulmonary tuberculosis in antiretroviral therapy programmes in low- and middle-income countries: A multicohort study: J Int AIDS Soc, 2019; 22(9); e25392
16. Huo ZY, Peng L, Is Xpert MTB/RIF appropriate for diagnosing tuberculous pleurisy with pleural fluid samples? A systematic review: BMC Infect Dis, 2018; 18(1); 284
17. Gopinath K, Singh S, Non-tuberculous mycobacteria in TB-endemic countries: Are we neglecting the danger: PLoS Negl Trop Dis, 2010; 4(4); e615
18. Helb D, Jones M, Story E: J Clin Microbiol, 2010; 48(1); 229-37
19. Zhao Y, Xu S, Wang L, National survey of drug-resistant tuberculosis in China: N Engl J Med, 2012; 366(23); 2161-70
20. Zong Z, Huo F, Shi J, Relapse versus reinfection of recurrent tuberculosis patients in a National Tuberculosis Specialized Hospital in Beijing, China: Front Microbiol, 2018; 9; 1858
21. Mesfin YM, Hailemariam D, Biadgilign S, Kibret KT, Association between HIV/AIDS and multi-drug resistance tuberculosis: A systematic review and meta-analysis: PLoS One, 2014; 9(1); e82235
22. Zürcher K, Reichmuth ML, Ballif M, Mortality from drug-resistant tuberculosis in high-burden countries comparing routine drug susceptibility testing with whole-genome sequencing: A multicentre cohort study: Lancet Microbe, 2021; 2(7); e320-e30
23. Kempker RR, Chkhartishvili N, Kinkladze I, High yield of active tuberculosis case finding among HIV-infected patients using Xpert MTB/RIF testing: Open Forum Infect Dis, 2019; 6(6); ofz233
24. Zifodya JS, Kreniske JS, Schiller I, Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis: Cochrane Database Syst Rev, 2021; 2; CD009593
25. Ngabonziza J, Decroo T, Migambi P, Prevalence and drivers of false-positive rifampicin-resistant Xpert MTB/RIF results: A prospective observational study in Rwanda: Lancet Microbe, 2020; 1(2); e74-e83
Figures
 Figure 1. Of the 413 HIV patients, 177 patients met the inclusion criteria, including those diagnosed with active PTB (145), and EPTB (32); 236 patients were excluded from the analysis. (PowerPoint, 2016 version, Microsoft).
Figure 1. Of the 413 HIV patients, 177 patients met the inclusion criteria, including those diagnosed with active PTB (145), and EPTB (32); 236 patients were excluded from the analysis. (PowerPoint, 2016 version, Microsoft). Figure 2. Receiver operating curves (ROC) for Xpert, Acid-fast bacilli (AFB) and T-SPOT.TB to differentiate tuberculosis (TB) and non-TB cases. AUC, area under the ROC. (SPSS, 21 version, Microsoft).
Figure 2. Receiver operating curves (ROC) for Xpert, Acid-fast bacilli (AFB) and T-SPOT.TB to differentiate tuberculosis (TB) and non-TB cases. AUC, area under the ROC. (SPSS, 21 version, Microsoft). Tables
 Table 1. Demographic characteristics of enrolled patients
Table 1. Demographic characteristics of enrolled patients Table 2. Performance of Xpert, AFB and T-SPOT in detecting PTB, EPTB and total cases.
Table 2. Performance of Xpert, AFB and T-SPOT in detecting PTB, EPTB and total cases. Table 3. Sensitivity and specificity of Xpert in different specimens.
Table 3. Sensitivity and specificity of Xpert in different specimens. Table 4. Comparison of the performance of Xpert, AFB and T-SPOT.TB in the diagnosis of pulmonary tuberculosis when CD4+ <200 cells/mm3.
Table 4. Comparison of the performance of Xpert, AFB and T-SPOT.TB in the diagnosis of pulmonary tuberculosis when CD4+ <200 cells/mm3. Table 1. Demographic characteristics of enrolled patients
Table 1. Demographic characteristics of enrolled patients Table 2. Performance of Xpert, AFB and T-SPOT in detecting PTB, EPTB and total cases.
Table 2. Performance of Xpert, AFB and T-SPOT in detecting PTB, EPTB and total cases. Table 3. Sensitivity and specificity of Xpert in different specimens.
Table 3. Sensitivity and specificity of Xpert in different specimens. Table 4. Comparison of the performance of Xpert, AFB and T-SPOT.TB in the diagnosis of pulmonary tuberculosis when CD4+ <200 cells/mm3.
Table 4. Comparison of the performance of Xpert, AFB and T-SPOT.TB in the diagnosis of pulmonary tuberculosis when CD4+ <200 cells/mm3. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








