22 July 2022: Database Analysis
Role of Serum Creatinine Levels in Prognostic Risk Stratification of Prostate Cancer Patients
Xingbo Gu1ACDEFG*, Jiaojiao Wu1BCDEF, Xuefeidan Liu2AD, Yun Hong1BCDF, Yaoxi Wu3AD, Ye Tian1ADDOI: 10.12659/MSM.937100
Med Sci Monit 2022; 28:e937100
Abstract
BACKGROUND: Studies on the relationship between serum creatinine and the prognosis of prostate cancer have been very limited. The aim of this study was to investigate the role of serum creatinine in the prognostic risk stratification of patients with prostate cancer.
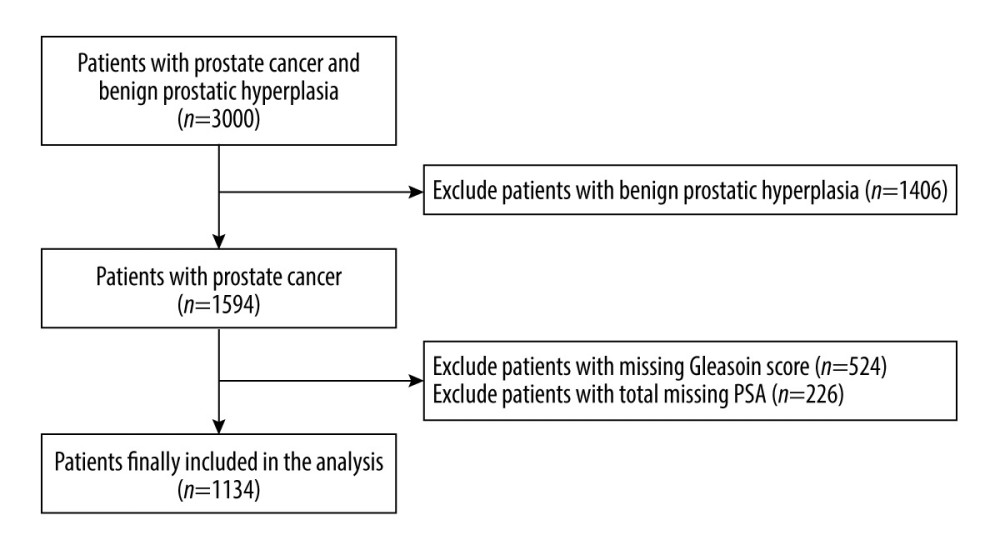
MATERIAL AND METHODS: We identified 1134 eligible patients from the “Prostate Cancer Data Set” in the National Clinical Medical Science Data Center. Patients with prostate cancer were divided high- and low-risk prognostic groups according to prostate-specific antigen levels and Gleason scores and were divided into 5 groups according to serum creatinine quintile: Q1 (<70.1 umol/L), Q2 (70.1-76.8 umol/L), Q3 (76.8-83.4 umol/L), Q4 (83.4-92.1 umol/L), and Q5 (>92.1 umol/L). Multivariate logistic regression and a multiple restricted cubic spline method were used to evaluate the relationship between serum creatinine level and the level of prostate cancer prognostic risk.
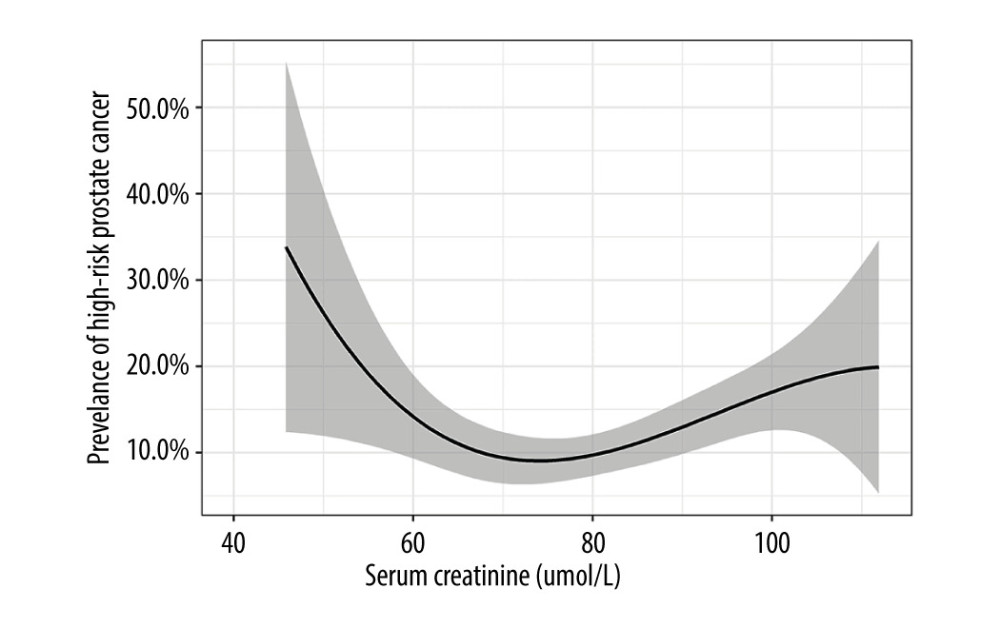
RESULTS: Of the 1134 patients with prostate cancer, 134 (11.8%) had a high-risk prognosis. Compared with the Q2 group (the reference group), the lowest serum creatinine levels in the Q1 group and the highest serum creatinine levels in groups Q5, Q3, and Q4 were associated with a high-risk prognosis, and this association remained significant after adjusting for confounders. The multiple restricted cubic spline regression model showed the relationship between serum creatinine level and high-risk prognosis was U-shaped.
CONCLUSIONS: Serum creatinine level was an independent predictor of high-risk prognosis. Controlling serum creatinine levels between 70.1 and 76.8 umol/L in patients with prostatic cancer may benefit the prognosis of patients with prostatic cancer.
Keywords: Creatinine, Prostatic Neoplasms, Prognosis, Risk Factors, Humans, Male, Neoplasm Grading, Risk Assessment
Background
Prostate cancer is a common genitourinary malignancy in men, and it is the second most common cancer worldwide and the fifth leading cause of cancer-related death in this sex [1]. According to the Centers for Disease Control, African Americans have the highest incidence of prostate cancer, followed by Europeans, and Asian ethnic groups have the lowest [2]. The incidence of prostate cancer in China has been increasing significantly in recent years. In 2015, the incidence of prostate cancer in China ranked sixth among male malignant tumors, and the death rate among male malignant tumors ranked tenth [3,4].
Early prostate cancer may not show associated symptoms, as its incubation period is imperceptible in development [5]. Most patients with prostate cancer are in the middle and advanced stages at the time of diagnosis, resulting in the overall prognosis of patients with prostate cancer in China being much lower than that in Western Europe and the United States [6]. Treatment modalities also vary widely among patients with different types of prostate cancer [7]. Patients with early, low-risk prostate cancer can achieve good therapeutic effects by radical surgery or radical radiotherapy and can even be cured [8]. However, patients with advanced, high-risk prostate cancer generally chose palliative treatment based on androgen deprivation therapy to prolong their survival [9]. The 2020 European Urological Association Prostate Cancer Diagnosis and Treatment Guidelines and the National Comprehensive Cancer Network Prostate Cancer Clinical Practice Guidelines point out that patients with different types of prostate cancer have different treatment modalities and also have different prognoses [10,11].
Serum creatinine is a metabolite of human muscle, which is closely related to the total amount of muscle in the body. The determination of serum creatinine concentration is an effective indicator for evaluating the glomerular filtration rate (GFR), which is important for clinical diagnosis and treatment [12]. In recent years, studies have shown that serum creatinine levels are correlated with serum-free prostate-specific antigen (PSA) levels, and changes in renal function can affect PSA [13]. Moreover, serum creatinine has been identified to be of clinical value in the diagnosis or prognosis of various neoplastic diseases, such as pancreatic cancer, vulvar cancer, and epithelial ovarian cancer [14–17].
At present, accurately distinguishing high-risk from low-risk patients in the clinical work of prostate cancer is one of the intractable problems facing clinicians and has an important impact on the treatment and prognostic measures of patients. However, studies on the prognostic role of serum creatinine in patients with prostate cancer are relatively scarce. Therefore, the primary aim of this study was to investigate the role of serum creatinine levels in the prognostic risk stratification of patients with prostate cancer to provide direction for clinical decision-making.
Material and Methods
STATISTICAL ANALYSIS:
Continuous variables were expressed as mean±standard deviation, and the independent sample
ETHICS:
This study was based on publicly available data from the “Prostate Cancer Data Set” in the National Population Health Data Center and did not involve interaction with human participants or the use of personally identifiable information. The study did not require informed consent, and the authors obtained a data use agreement from the National Clinical Medical Science Data Center.
Results
Among the 1134 patients with prostate cancer, 134 (11.8%) had a high-risk prognosis. Their mean age was 66.9 years, with a standard deviation of 8.0 years; and the mean BMI was 24.9 kg/m2, with a standard deviation of 3.0 kg/m2. The results showed that the levels of alkaline phosphatase, creatine kinase isoenzyme, calcium, lactate dehydrogenase, and creatinine in the high-risk group were significantly higher than those in the low-risk group, while the levels of serum albumin, inorganic phosphorus, low-density lipoprotein cholesterol, and apolipoprotein A1 were higher in the low-risk group. The specific results are shown in Table 1.
In the unadjusted logistic regression model (Table 2), with serum creatinine group
The results of the subgroup analysis showed that there was no significant heterogeneity between serum creatinine level and a high-risk prognosis (
Discussion
Based on data from 1134 patients with prostate cancer, the main finding of this study was that serum creatinine was an independent risk factor for a high-risk prostate cancer prognosis, and the relationship showed a U-shaped curve. Patients with decreased and increased serum creatinine levels have a significantly higher prognostic risk of prostate cancer. The prognostic risk of prostate cancer was lowest when serum creatinine levels were in the range of 70.1–76.8 umol/L. In the different adjusted models, the groups were consistent with the associations obtained before adjustment.
At present, there are very few studies on the relationship between serum creatinine and the prognosis of prostate cancer. A relevant domestic study has shown that the combined detection of sarcosine levels and matrix metallopeptidase 9 has diagnostic value for prostate cancer, and sarcosine is involved in the cancerous process of the prostate [20]. Moreover, in another study involving 262 patients with prostatic diseases, it was shown that sarcosine showed an increase during prostate cancer; however, it could also reflect the degree of invasion of prostate cancer [21]. The results of a case-control study in Nigeria showed that the increase in serum urea and creatinine concentrations and the decrease in cystatin C levels can increase the risk of renal dysfunction in patients with prostate cancer [22]. These studies suggest a possible implication of serum creatinine in the prognostic risk stratification in prostate cancer. After 1134 patients with prostate cancer in the present study were adjusted for age, BMI, serum albumin, alkaline phosphatase, calcium, and other risk factors, lower or higher serum creatinine levels were significantly associated with a high-risk prostate cancer prognosis. Our findings suggest an independent predictive role of serum creatinine in prostate cancer prognosis and suggest that controlling serum creatinine concentrations in patients with prostate cancer at 70.1 to 76.8 umol/L may have positive significance for patient prognosis.
Additional studies have been on the role of creatine and sarcosine in the development of prostate cancer and the role of serum creatinine in the diagnosis of prostate cancer [23,24]. However, there are very few studies on the relationship between serum creatinine and the prognostic risk of prostate cancer, and the exact mechanism is still unclear. Therefore, based on previous studies, we proposed the following hypothesis about the mechanism of the relationship between serum creatinine and high-risk prostate cancer prognosis: Serum creatinine levels can reflect the nutritional, metabolic, and renal status of patients to some extent [25]. Clinically, a decrease in serum creatinine indicates that the patient’s total muscle mass is reduced and malnourished, while an increase indicates impaired renal function [26]. Serum creatinine is mainly derived from the nonenzymatic conversion of creatine and phosphocreatine [27]. Most of the creatine and phosphocreatine are produced by muscle, and creatinine is excreted in the urine almost exclusively after renal filtration [28]. Some studies have shown that a too-low level of serum creatinine in malignant tumors affects the prognosis of patients [29]. They concluded that the decrease in serum creatinine in patients with malignant tumors is due to the presence of varying degrees of cachexia, resulting in thin muscles, hypoproteinemia, and severe malnutrition, which may lead to reduced survival time and poor prognosis [30]. Moreover, there are large differences in the metabolism between tumor cells and normal human cells [31]. In view of the rapid growth characteristics of tumor cells, more nutrients and adenosine triphosphate (ATP) conversion processes are often required to maintain their high metabolism and rapid growth characteristics [32]. Sarcosine plays an important role in the process of ATP metabolism [33]. Many studies have shown that cancer cells have increased sarcosine concentrations at high metabolic levels [34].
Sarcosine is the main amino acid for the synthesis of creatine and creatinine. A high concentration of sarcosine will lead to increased serum creatinine production, which also confirms the role of serum creatinine in cancer. Finally, studies have shown that blood lipids and lipoproteins are positively correlated with the high risk of prostate cancer [35]. Blood lipids are important factors affecting serum creatinine and therefore low levels of serum creatinine are mainly due to the low nutritional status of patients with prostate cancer, which affects patient prognosis [36]. High levels of serum creatinine can be involved in the conversion of nutrients and ATP in the growth of prostate tumor cells and the role of blood lipids and lipoproteins in the development of prostate cancer, resulting in an increased prevalence of prostate cancer and poor prognosis of prostate cancer patients.
Our study had limitations. First, the data of this study were obtained from a single center of the National Center for Clinical Medical Sciences (the Chinese PLA General Hospital), and the results were not as representative as those of a multicenter study. Second, we did not directly participate in the collection of study data and therefore there may be some limiting factors in the study. Third, there were some missing data on PSA and Gleason scores in the study. These patients were excluded from the data cleansing phase, which may have led to selection bias. Fourth, creatinine also reflects renal function to a degree, so preoperative renal function is an important parameter that may directly effect the prognosis of prostate cancer. In addition, prostate cancer patients with high Gleason scores can have urinary tract metastasis, which may also have had some influence on the results. Unfortunately, the dataset we requested does not have information on these 2 factors. Fifth, although confounding factors such as age and BMI were adjusted for in the multivariate model, it cannot be ruled out that other unmeasured or inadequately measured factors may have confounded the true association. Sixth, through data analysis, we concluded that the serum creatinine level has an effect on the prognostic risk of prostate cancer, but this study is a retrospective clinical observational study, and its results do not explain the causal relationship between the 2, as in longitudinal studies. Therefore, more studies are needed to provide strong evidence for the association between these 2 variables.
Conclusions
Serum creatinine levels are independently associated with a high-risk prognosis in column adenocarcinoma. The mechanism of the relationship between serum creatinine and prognosis in prostate cancer patients is still lacking strong evidence. However, our study showed that there is a U-shaped curve relationship between serum creatinine levels and the level of prostate cancer prognosis. Low or high serum creatinine levels increase the prognostic risk of prostate cancer. Controlling the serum creatinine level at 70.1 to 76.8 umol/L in patients with prostate cancer may be beneficial for their prognosis.
Tables
Table 1. Characteristics of patients with prostate cancer.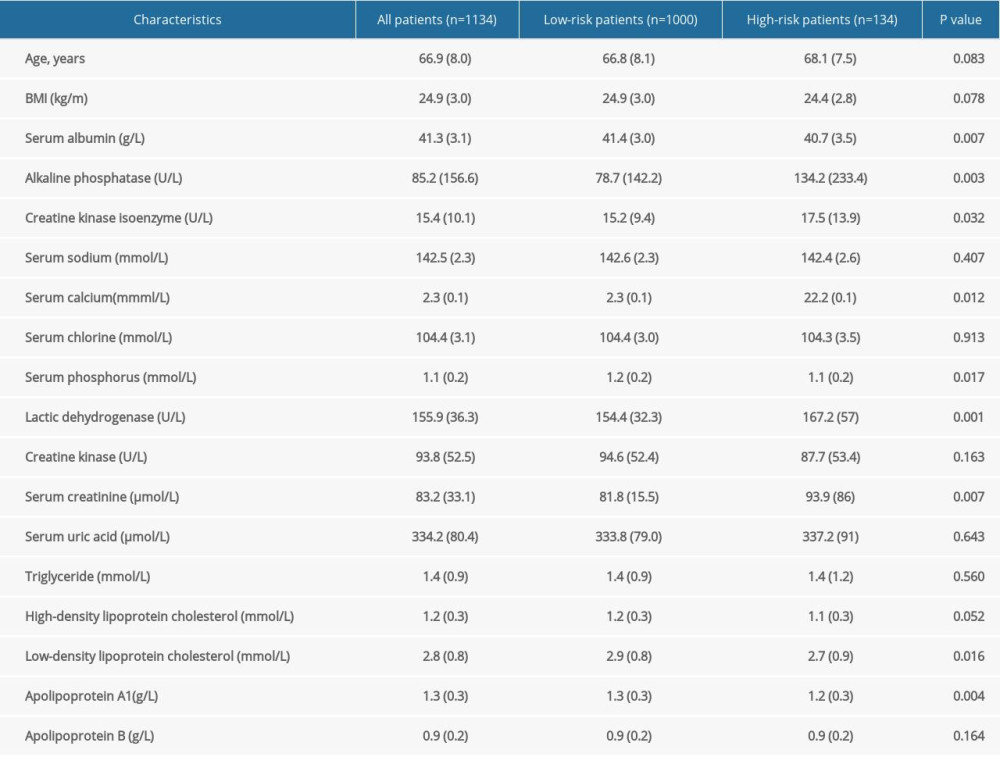 Table 2. Odds ratio and 95% confidence interval of 1134 patients with prostate cancer according to the quintiles of serum creatinine.
Table 2. Odds ratio and 95% confidence interval of 1134 patients with prostate cancer according to the quintiles of serum creatinine.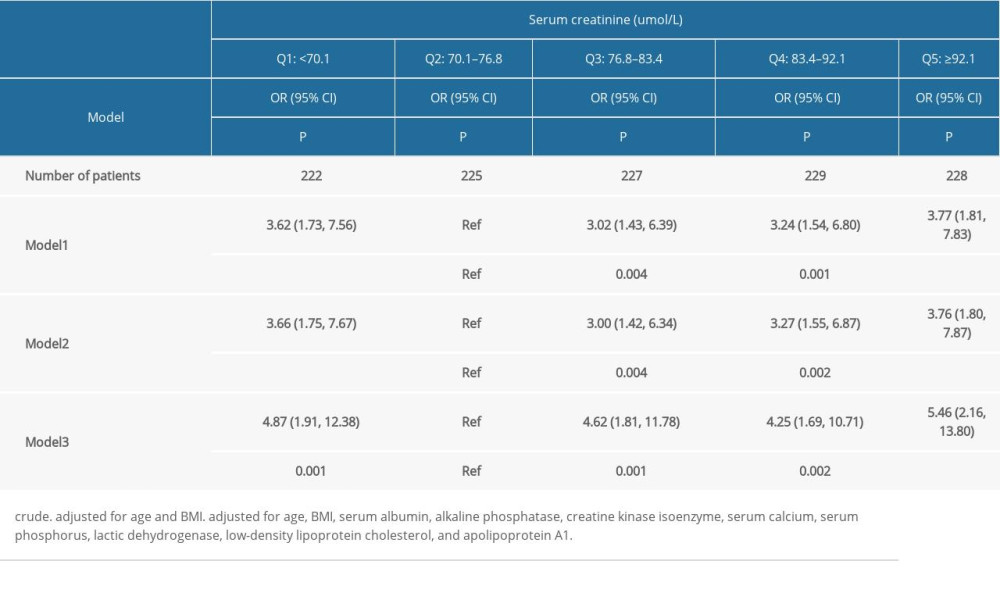 Table 3. Odds ratio and 95% confidence interval of high risk of prostatic cancer according to quintiles of creatinine: subgroup analyses.
Table 3. Odds ratio and 95% confidence interval of high risk of prostatic cancer according to quintiles of creatinine: subgroup analyses.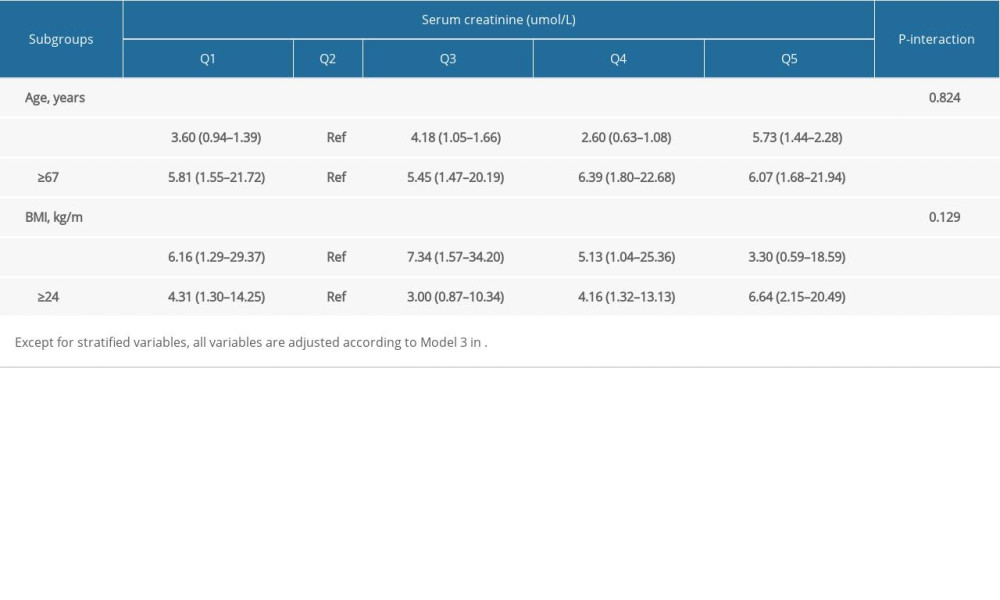
References
1. McGuire S: World cancer report 2014, 2015; 7(2); 418-19, Geneva, Switzerland, World Health Organization, international agency for research on cancer, WHO Press
2. Rebbeck TR, Prostate cancer genetics: Variation by race, ethnicity, and geography: Semin Radiat Oncol, 2017; 27(1); 3-10
3. Liu X, Yu C, Bi Y, Zhang Z, Trends and age-period-cohort effect on incidence and mortality of prostate cancer from 1990 to 2017 in China: Public Health, 2019; 172; 70-80
4. Zhang S, Sun K, Zheng R, Cancer incidence and mortality in China, 2015: J Natl Cancer Center, 2021; 1(1); 2-11
5. Van Poppel H, Roobol MJ, Chapple CR, Prostate-specific antigen testing as part of a risk-adapted early detection strategy for prostate cancer: European Association of Urology position and recommendations for 2021: Eur Urol, 2021; 80(6); 703-11
6. Xu L, Wang J, Guo B, Comparison of clinical and survival characteristics between prostate cancer patients of PSA-based screening and clinical diagnosis in China: Oncotarget, 2018; 9(1); 428
7. Tian J-Y, Guo F-J, Zheng G-Y, Ahmad A, Prostate cancer: Updates on current strategies for screening, diagnosis and clinical implications of treatment modalities: Carcinogenesis, 2018; 39(3); 307-17
8. Costello AJ: Nat Rev Urol, 2020; 17(3); 177-88
9. Nabid A, Carrier N, Martin A-G, Duration of androgen deprivation therapy in high-risk prostate cancer: A randomized phase III trial: Eur Urol, 2018; 74(4); 432-41
10. Parker C, Castro E, Fizazi K, Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up: Ann Oncol, 2020; 31(9); 1119-34
11. Schaeffer E, Srinivas S, Antonarakis ES, NCCN guidelines insights: Prostate cancer, version 1.2021: Featured updates to the NCCN guidelines: J Natl Compr Canc Netw, 2021; 19(2); 134-43
12. Rule AD, Larson TS, Bergstralh EJ, Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease: Ann Intern Med, 2004; 141(12); 929-37
13. Weinstein SJ, Mackrain K, Stolzenberg-Solomon RZ, Serum creatinine and prostate cancer risk in a prospective study: Cancer Epidemiol Biomarkers Prev, 2009; 18(10); 2643-49
14. Zhang D-X, Dai Y-D, Yuan S-X, Tao L, Prognostic factors in patients with pancreatic cancer: Exp Ther Med, 2012; 3(3); 423-32
15. Malyszko J, Tesarova P, Capasso G, Capasso A, The link between kidney disease and cancer: Complications and treatment: Lancet, 2020; 396(10246); 277-87
16. Schwameis R, Postl M, Bekos C, Prognostic value of serum creatine level in patients with vulvar cancer: Sci Rep, 2019; 9(1); 11129
17. Lafleur J, Hefler-Frischmuth K, Grimm C, Prognostic value of serum creatinine levels in patients with epithelial ovarian cancer: Anticancer Res, 2018; 38(9); 5127-30
18. D’Amico AV, Whittington R, Kaplan I, Calculated prostate carcinoma volume: The optimal predictor of 3-year prostate specific antigen (PSA) failure free survival after surgery or radiation therapy of patients with pretreatment PSA levels of 4–20 nanograms per milliliter: Cancer, 1998; 82(2); 334-41
19. Kanehira M, Takata R, Ishii S, Predictive factors for short-term biochemical recurrence-free survival after robot-assisted laparoscopic radical prostatectomy in high-risk prostate cancer patients: Int J Clin Oncol, 2019; 24(9); 1099-104
20. Han H, Zhan Z, Xu J, Song Z, TMEFF2 inhibits pancreatic cancer cells proliferation, migration, and invasion by suppressing phosphorylation of the MAPK signaling pathway: OncoTargets Ther, 2019; 12; 11371
21. Narwal V, Kumar P, Joon P, Pundir C, Fabrication of an amperometric sarcosine biosensor based on sarcosine oxidase/chitosan/CuNPs/c-MWCNT/Au electrode for detection of prostate cancer: Enzyme Microb Technol, 2018; 113; 44-51
22. Oluboyo A, Adeleke A, Oluboyo B, Evaluation of selected renal markers in prostate cancer: J Appl Sci and Environ Manag, 2019; 23(9); 1725-28
23. Amamoto R, Uchiumi T, Yagi M, The expression of ubiquitous mitochondrial creatine kinase is downregulated as prostate cancer progression: J Cancer, 2016; 7(1); 50
24. Cernei N, Heger Z, Gumulec J, Sarcosine as a potential prostate cancer biomarker – a review: Int J Mol Sci, 2013; 14(7); 13893-908
25. Pupim LB, Cuppari L, Ikizler TA, Nutrition and metabolism in kidney disease: Semin Nephrol, 2006; 26(2); 134-57
26. Heimbürger O, Qureshi AR, Blaner WS, Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy: Am J Kidney Dis, 2000; 36(6); 1213-25
27. Kashani K, Rosner MH, Ostermann M, Creatinine: From physiology to clinical application: Eur J Intern Med, 2020; 72; 9-14
28. Braun J-P, Lefebvre H, Watson A, Creatinine in the dog: A review: Vet Clin Pathol, 2003; 32(4); 162-79
29. Poon RT, Fan ST, Lo CM, Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: Analysis of 1222 consecutive patients from a prospective database: Ann Surg, 2004; 240(4); 698
30. Hall JC, Nutritional assessment of surgery patients: J Am Coll Surg, 2006; 202(5); 837-43
31. Bergers G, Fendt S-M, The metabolism of cancer cells during metastasis: Nat Rev Cancer, 2021; 21(3); 162-80
32. Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Cancer metabolism: A therapeutic perspective: Nat Rev Clin Oncol, 2017; 14(1); 11-31
33. de Andrade RB, Gemelli T, Rojas DB, Evaluation of oxidative stress parameters and energy metabolism in cerebral cortex of rats subjected to sarcosine administration: Mol Neurobiol, 2017; 54(6); 4496-506
34. Petersen LF, Brockton NT, Bakkar A, Elevated physiological levels of folic acid can increase in vitro growth and invasiveness of prostate cancer cells: BJU Int, 2012; 109(5); 788-95
35. Allott EH, Howard LE, Cooperberg MR, Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database: Cancer Epidemiol Biomarkers Prev, 2014; 23(11); 2349-56
36. Droz J-P, Balducci L, Bolla M, Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults: Crit Rev Oncol Hematol, 2010; 73(1); 68-91
Figures
Tables
 Table 1. Characteristics of patients with prostate cancer.
Table 1. Characteristics of patients with prostate cancer. Table 2. Odds ratio and 95% confidence interval of 1134 patients with prostate cancer according to the quintiles of serum creatinine.
Table 2. Odds ratio and 95% confidence interval of 1134 patients with prostate cancer according to the quintiles of serum creatinine. Table 3. Odds ratio and 95% confidence interval of high risk of prostatic cancer according to quintiles of creatinine: subgroup analyses.
Table 3. Odds ratio and 95% confidence interval of high risk of prostatic cancer according to quintiles of creatinine: subgroup analyses. Table 1. Characteristics of patients with prostate cancer.
Table 1. Characteristics of patients with prostate cancer. Table 2. Odds ratio and 95% confidence interval of 1134 patients with prostate cancer according to the quintiles of serum creatinine.
Table 2. Odds ratio and 95% confidence interval of 1134 patients with prostate cancer according to the quintiles of serum creatinine. Table 3. Odds ratio and 95% confidence interval of high risk of prostatic cancer according to quintiles of creatinine: subgroup analyses.
Table 3. Odds ratio and 95% confidence interval of high risk of prostatic cancer according to quintiles of creatinine: subgroup analyses. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952