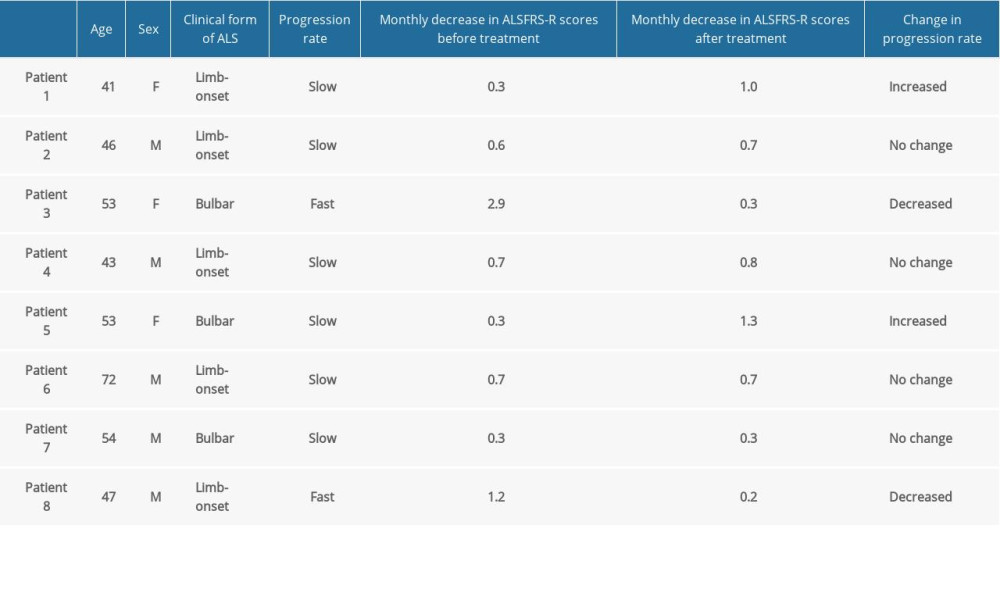
10 December 2020: Clinical Research
Repeat Administration of Bone Marrow-Derived Mesenchymal Stem Cells for Treatment of Amyotrophic Lateral Sclerosis
Tomasz Siwek12ABDEFG*, Katarzyna Jezierska-Woźniak3ABDEF, Stanisław Maksymowicz4BCDEF, Monika Barczewska56BD, Mariusz Sowa57B, Wanda Badowska8B, Wojciech Maksymowicz57ADGDOI: 10.12659/MSM.927484
Med Sci Monit 2020; 26:e927484
Abstract
BACKGROUND: The aim of this study was to investigate repeated intrathecal injection of autologous bone marrow-derived mesenchymal stem cells (BM-D MSCs) to patients for treatment of sporadic amyotrophic lateral sclerosis (ALS).
MATERIAL AND METHODS: Autologous MSCs were isolated from the patients’ bone marrow, plated, expanded, harvested, and passaged. Stem cells from a single bone marrow collection were used for 3 injections per patient, given over a 3-month period. Outcomes were measured with the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Participants were observed for a minimum of 6 months before transplantation to assess the natural course of ALS and for the same amount of time after transplantation to compare the rate of disease progression, estimated based on average monthly changes in ALSFRS-R scores. Data from 8 of the 15 participants eligible for the study were analyzed.
RESULTS: The safety of the MSC injections was confirmed and various effects of the therapy were documented. In patients who had ALS with an inherently slow course, there were no significant changes in the rate of disease progression. In patients who had ALS with an inherently rapid course, slowing of the disease was noted following treatment with MSCs. However, because that subgroup was so small, it was not possible to assess whether the changes were statistically significant.
CONCLUSIONS: Identifying groups of patients who are not responding or potentially responding negatively to injection of MSCs may help prevent it from being offered to individuals who may not benefit from the therapy. One of the limitations of this treatment method is the amount of time required for long-lasting preparation of bone marrow-derived MSCs for a disease that is rapidly progressive. Therefore, it is worth looking for other allogeneic sources of stromal cells for these types of injections.
Keywords: Amyotrophic Lateral Sclerosis, Injections, Spinal, Mesenchymal Stem Cell Transplantation, Disease Progression, Mesenchymal Stem Cells
Background
Amyotrophic lateral sclerosis (ALS) is an uncommon neurodegenerative disease that is rapidly fatal. The main symptoms – limb weakness, muscle atrophy, disturbance in speaking and swallowing, and respiration insufficiency – are a result of motor neuron damage. Riluzole and edaravone are the only 2 drugs approved for ALS [1]. Therefore, there is a great need for new treatment options.
Since 2003, attempts have been made to use mesenchymal stem cells (MSCs) to stop the degenerative process of neurons [2]. Different sources of MSCs have been tested in many trials, and different forms of administration (intramuscular, intravenous, intracerebral, and intrathecal) [3–5]. The authors of these studies have consistently noted the relative safety of the therapy and a transient clinical effect in slowing the natural course of ALS [6–9]. Given these results, an obvious extension of the research would be to perform repeated stem cell transplantation and assess the effects of treatment over time.
Our previous study [10] showed the possible existence of a group of patients who responded to a single administration of MSCs. Inspired by those outcomes, we conducted a study of repeated administration of MSC from the same source, using the same procedures for preparation and administration as in the earlier research, to confirm the transient effect that we had reported and to verify the presence of the responder group.
Material and Methods
PARTICIPANTS AND INCLUSION CRITERIA:
Patients between 18 and 65 years old with a definitive diagnosis of sporadic ALS, according to the El Escorial criteria, and a force vital capacity ≥50% were enrolled in the study. Individuals who had inflammatory disorders; active infections; an active neoplastic process in the last 5 years; tested positive test for HIV, hepatitis C, hepatitis B, or
Fifteen patients with a mean age of 54.06 years and a mean Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) score of 38.7 points were enrolled. Three patients presented with bulbar signs on enrollment and 12 had limb onset ALS.
MSC PREPARATION AND ADMINISTRATION AND PROCEDURE FOLLOW-UP:
Autologous MSCs, which have plastic-adherent properties in tissue culture, were isolated from the patients’ bone marrow under sterile conditions. Nucleated cells were collected, diluted with 2 volumes of phosphate-buffered saline, centrifuged twice at 100G for 10 minutes, and finally resuspended in culture medium [11].
Cells were plated, expanded, and grown at 37°C and 5% CO2, until they reached confluency, then harvested and passaged (≤30 days in culture and 2 passages). The MSC samples were tested with a fluorescence-activated cell sorter (BD FACSAria II) for features described in the International Society for Cellular Therapy guidelines [12].
Following standard lumbar puncture, the MSCs were intrathecally injected into each patient 3 times, at 3-month intervals. For the procedure, 2 mL of cerebrospinal fluid (CSF) was collected and the same volume of BM-MSC solution, containing a mean of 10×106 cells, was injected with a needle into the spinal canal. The same neurosurgeon performed all of the treatments.
In 2 patients, the third administration contained allogenic Wharton’s jelly-derived MSCs because the patients’ own bone marrow had become depleted. In 2 other patients, for humanitarian reasons, the decision was made to withdraw from the next invasive bone marrow collection and to complete MSC delivery from another source. Another 3 patients terminated the therapy after the second MSC injection. One other patient remained stable from the beginning of the trial, with tetraplegia and mutism, and had consistently low scores on the functional scale.
METHODS OF ASSESSING OUTCOME:
For outcome measurement in ALS, ALSFRS-R has been used. It is a revised functional rating scale that reflects disease status and level of disability specifically in patients with ALS. The scale is based on the answers to 12 questions, which assess functions of speech and swallowing that correspond to bulbar ability, self-care, and patient mobility as it applies to performing gross and fine motor tasks, and expanded respiratory function [13]. The answers correspond to scores from 0 to 4, where 0 is the largest deficit in a given area and 4 is lack of a deficit. The total score ranges from 0 to 48 points. ALSFRS-R scores show a linear progressive decline in the natural course of the disease [14]. The methodological experience gained in this and other research has supported the process of validating the ALSFRS-R scale for use in medical experiments in Poland [15].
To estimate the baseline rate of disease progression in each patient, the participants were observed for at least 6 months before treatment.
Telephone interviews were conducted with the patients to review the questionnaires for the ALSFRS-R scale. All of them were interviewed by the same rater neurologist every 2 months. Patients who received MSCs were followed up with observation for at least 6 months. One patient who had only a single ALSFRS-R score after treatment was excluded. The results from 8 patients were analyzed.
STATISTICAL ANALYSIS:
Patients who received at least 3 injections of MSCs and were assessed at least 3 times after beginning treatment were included in the analysis. Data for the statistical analysis were from 3 women and 5 men. Their median age was 53 years (range 41–72 years; interquartile range 44.5–55 years).
Five patients had the limb onset form of ALS and 3 had the bulbar form of the disease. All patients were also treated with riluzole. Two participants had disease that was rapidly progressive (loss >1.0 point per month on ALS-FRS-R) and 6 had disease that was slowly progressive (loss <1 point per month on ALS-FRS-R). When the patients were evaluated, their predicted ALSFRS-R scores were estimated using linear regression, in part because the intervals at which some of them received MSCs varied, for reasons such as infection or other random situations. The average monthly decrease in ALSFRS-R score for the 6-month period before and after treatment was calculated. In all cases,
Results
SAFETY:
The safety of treatment with MSCs was assessed by regularly checking the clinical state of patients and analyzing adverse events (AEs) during every transplantation. Patients were informed about the necessity of immediately reporting any medical events that occurred during the study, including their nature and severity, timing, and duration. Any AEs were assessed for their relationship with treatment.
None of the patients had AEs after bone marrow collection. However, in 1 patient, bone marrow aspiration was conducted twice because the biological material obtained during the first procedure was infected. No immediate or chronic surgical or local complications were observed after the MSC injections.
One patient had a post-dural puncture headache, which resolved spontaneously without complications or a need for medication. Moreover, no major AEs associated with bone marrow collection or lumbar puncture were reported by any of the patients during the 6-month follow-up period.
EFFICACY:
The differences between the monthly rate of progression (loss of points per month) before treatment versus during and after treatment, as assessed with the Wilcoxon test, were not statistically significant (P=0.57) (Figure 1). The reason for this is that in 2 cases (Patients 3 and 8), the monthly rate of progression decreased, but in 2 other cases (Patients 1 and 5), the rate increased. The rate of increase was unchanged in the other 4 patients. Patient 7 experienced a sharp decrease in score around the time of the first treatment, but there was no change in the rate of progression during the subsequent injections. Therefore, the initial decrease was probably not associated with the use of MSCs.
A detailed summary of the demographic data and disease rate indicators for the study participants is shown in the Table 1.
The dynamics of the natural course of ALS in individual patients before therapy and the modifications observed during and after treatment with MSCs are presented in Figures 2–4.
Because of the very small size of the group, only very limited subgroup analysis was possible (in subgroups with ≥5 participants). In the men, the differences in the rates of progression, based on ALSFRS-R scores, were not statistically significant (P=0.50). The same was true in the subgroup of patients with limb onset ALS (P=0.50). A significant difference was seen in patients with slowly progressive. Overall, the rate of progression did not decrease in this group, and in 2 patients, it was greater at the end of the study than during their first assessment. In this group, the median number of points lost per month increased from 0.47 before treatment to 0.76 after therapy (P=0.028) (Figure 5).
Discussion
Analysis, in different reviews, of studies of the use of MSCs for ALS shows that the treatment has a partial influence on the disease’s development [16–18]. Our team recognized that an important direction should be analysis of a group of patients who benefited from therapy with MSCs, a search for predictors of the clinical efficacy of use of autologous MSCs in ALS [19], and assessment of the results of repeated administration of the treatment.
The results of the first study assessing multiple intrathecal injections of autologous MSCs obtained from bone marrow were published in 2015, and follow-up of those patients was reported in 2018. The first assessment of 2 administrations in 8 patients confirmed the safety of the method [20].
Another study of a group of more than 30 patients and a comparable placebo control group showed that significantly more patients who received MSCs had declines in development of clinical features of ALS and in biochemical parameters used to assess the pathomechanism of the disease’s progression [21].
However, no direct comparison can be made between our results and those from the previous trials, if only because different methods of cell processing were used. It should also be pointed out that in our study, a single bone marrow collection from each patient was used. We performed the same method of cell culture for all 3 administrations. A larger number of injections means not only a long period of treatment but also a longer time for cell processing, which was a limitation of our study.
Moreover, because our study was nationwide, it included patients from distant cities, which meant repeated travel for them and a need for us to organize it to meet the requirements of individuals with disabilities. In future research, to ensure that participants cooperate with such arrangements, it is worth considering a logistical support program, which we did not have for the present study. Unfortunately, that turned out to be a serious limitation.
Finally, the small number of patients and the relatively high percentage of them who withdrew from the study are weaknesses of the research. Because of the sample size, fewer subgroups were available for statistical analysis, limiting our ability to draw conclusions from the data.
Conclusions
The response of patients with ALS in our study to treatment with MSCs was variable. The absence of significant AEs in the course of multiple intrathecal injections of MSCs was a favorable result. Patients who benefited from treatment had ALS that was rapidly progressive before therapy administration. However, because of the small sample size, it is not possible to draw a conclusion about whether the rate of progression prior to treatment is a factor predictive of good response to therapy with MSCs.
An important conclusion is the fact that the rate of ALS progression did not decrease after the administration of MSCs in patients with slowly progressive disease, and in 2 cases, it increased, which influenced our statistical conclusions.
The results of numerous studies show the transient beneficial clinical effect of intrathecal injection of MSCs. However, the efficacy of the clinical changes after treatment is not uniform among patients.
Identifying groups of patients fail to respond or are responding negatively to treatment with MSCs may preempt further testing of the method in patients who are not likely to benefit from the therapy. Subsequent studies in larger groups of patients with ALS may enable targeting of its use to the “responders” group, increase its safety and effectiveness.
Finally, we need to stress some limitations of use of autologous MSCs for therapy, particularly in patients with rapid-progressing diseases such as ALS. The preparation of bone marrow-derived MSCs is associated with many challenges for patients and it takes a lot of valuable time. For that reason, it is worth looking for other allogeneic sources of stromal cells for this type of therapy.
Figures
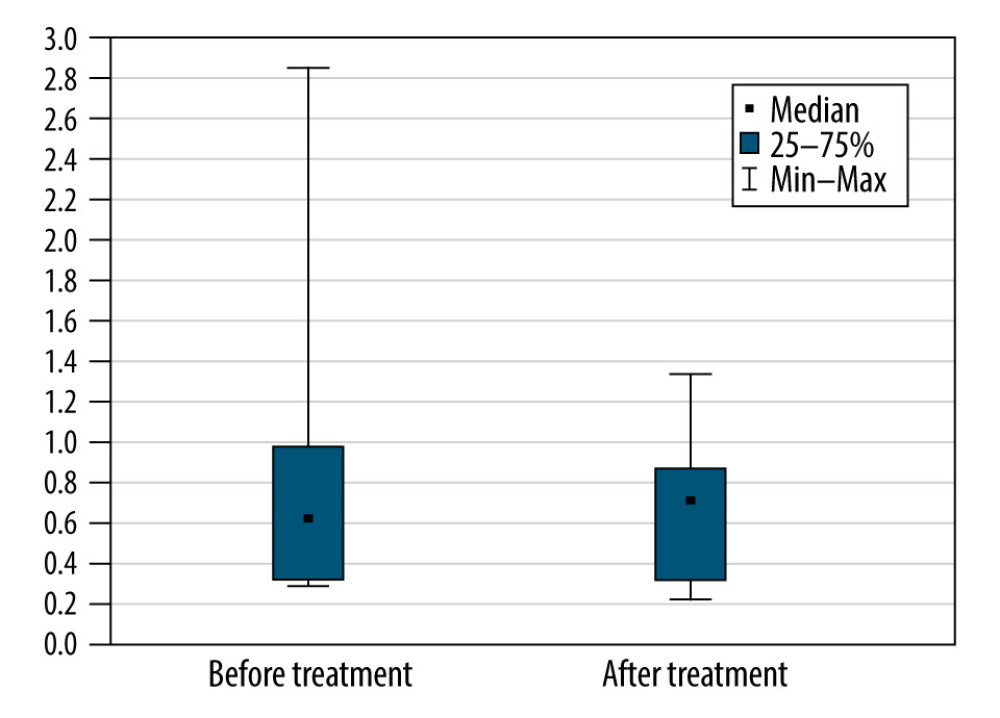 Figure 1. The monthly decrease in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and after treatment for all participants (P=0.57).
Figure 1. The monthly decrease in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and after treatment for all participants (P=0.57). 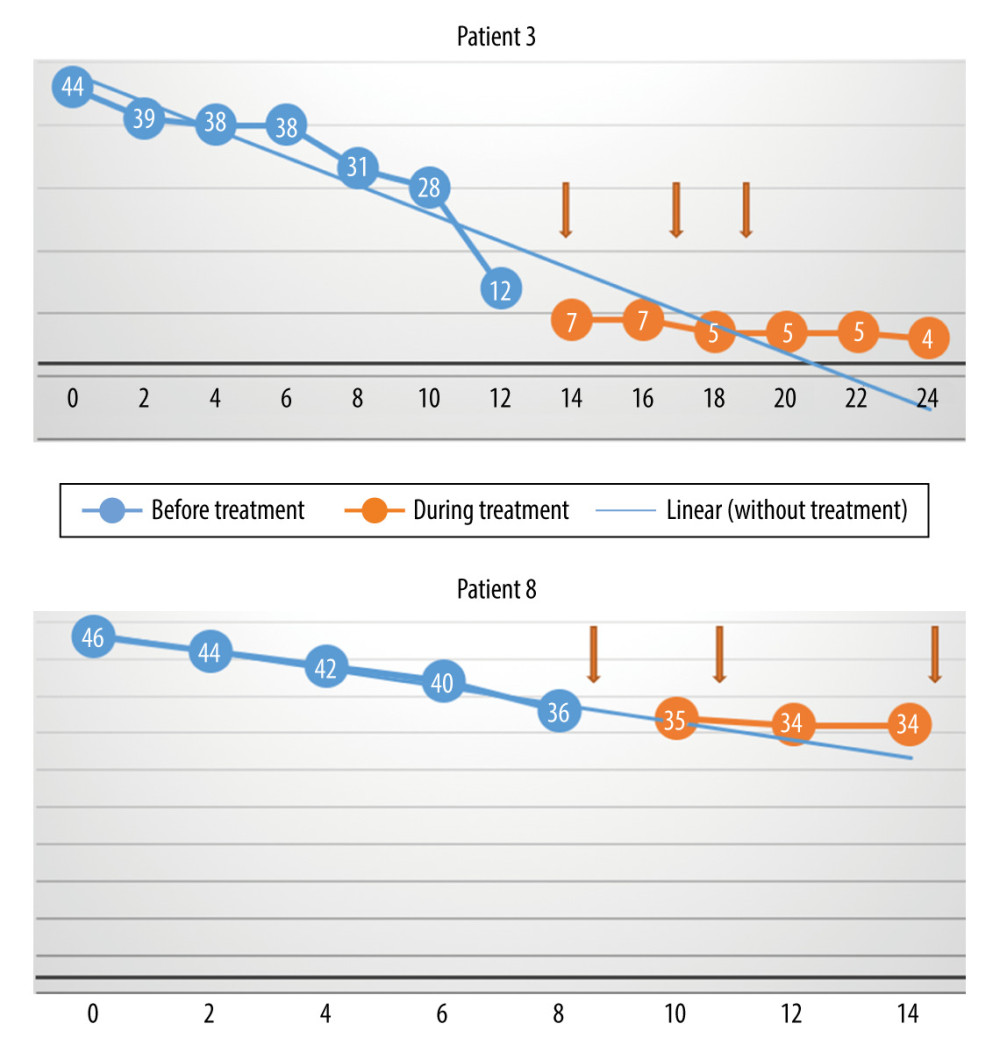 Figure 2. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period, based on data from the pretreatment observation period. The rate of progression decreased. (Arrows indicate injections.)
Figure 2. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period, based on data from the pretreatment observation period. The rate of progression decreased. (Arrows indicate injections.) 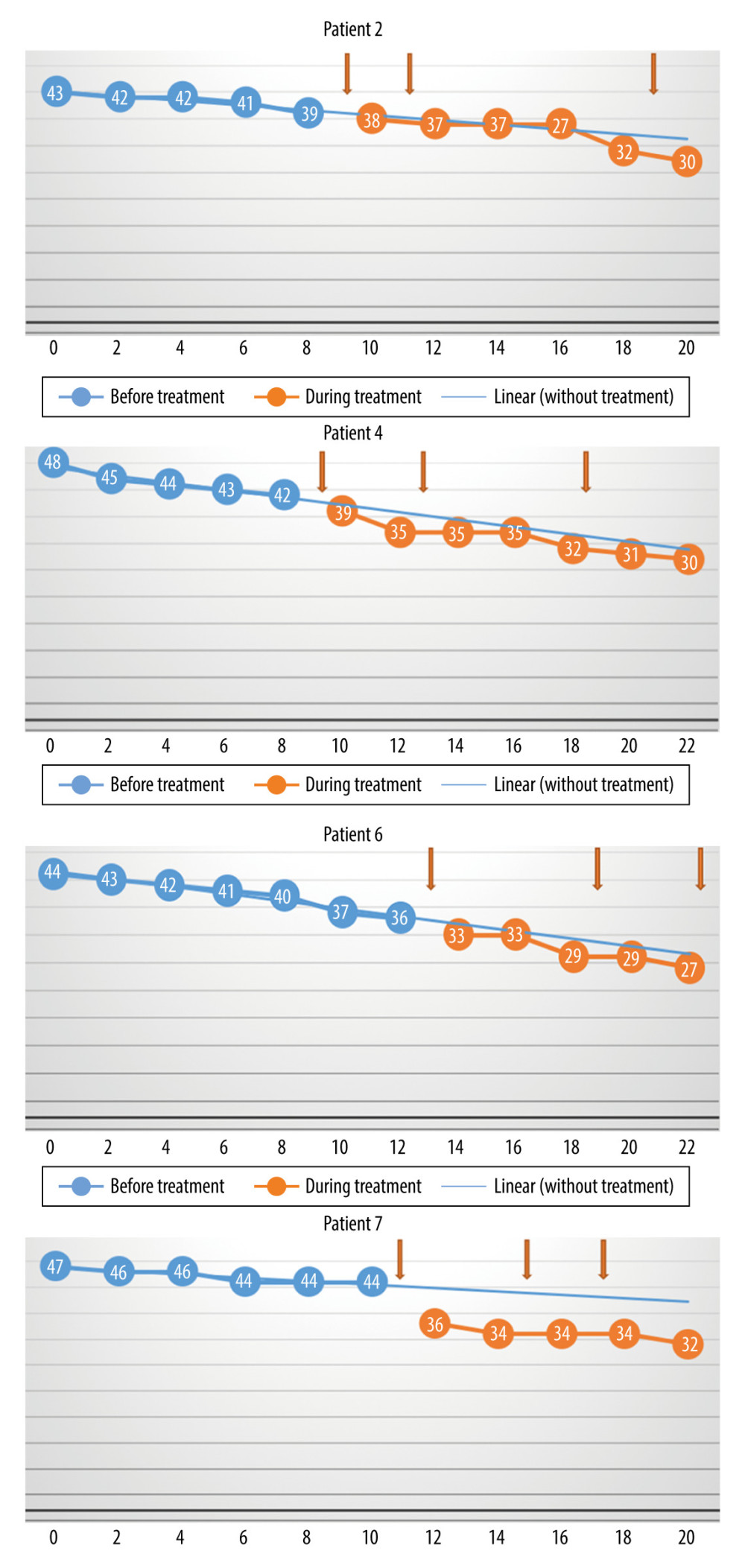 Figure 3. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period, based on data from the pretreatment observation period. The rate of progression was unchanged. (Arrows indicate injections.)
Figure 3. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period, based on data from the pretreatment observation period. The rate of progression was unchanged. (Arrows indicate injections.) 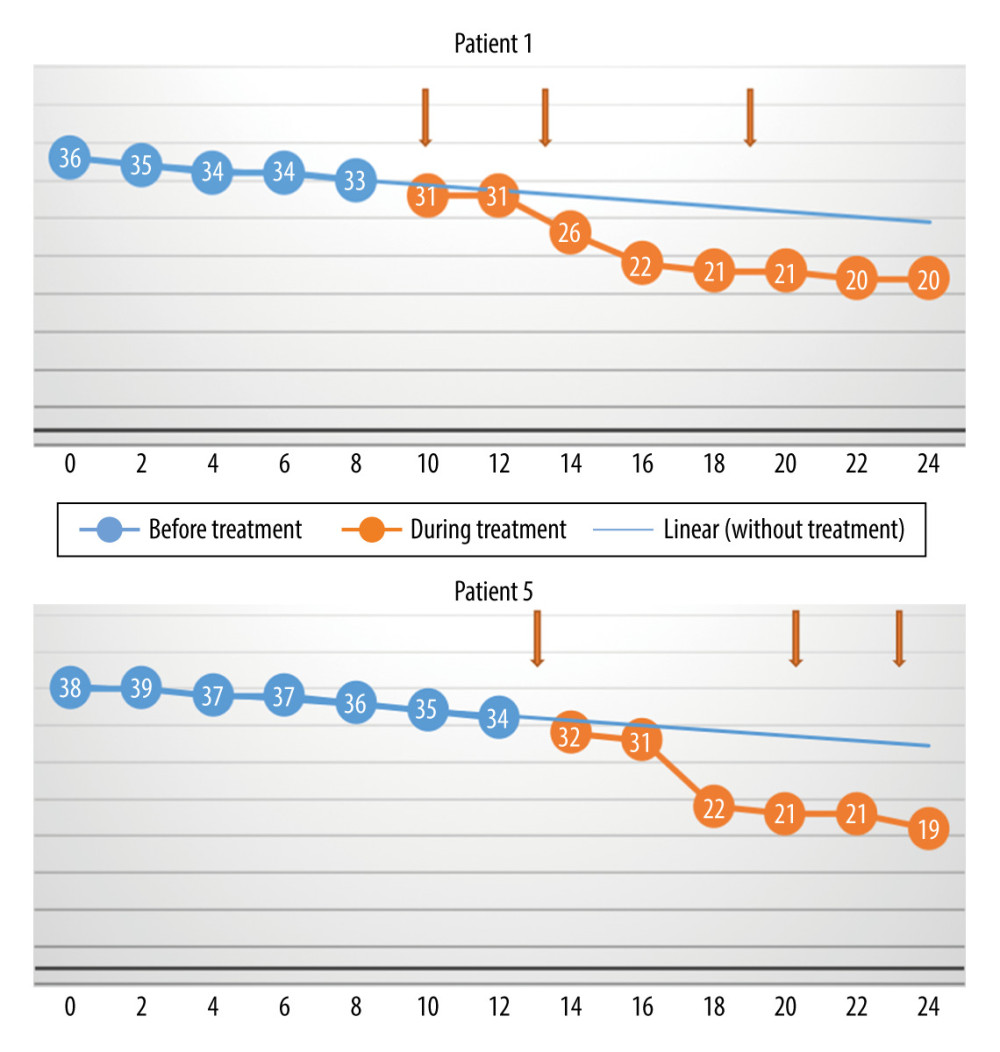 Figure 4. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period based on data from the pretreatment observation period. The rate of progression increased. (Arrows indicate injections.)
Figure 4. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period based on data from the pretreatment observation period. The rate of progression increased. (Arrows indicate injections.) 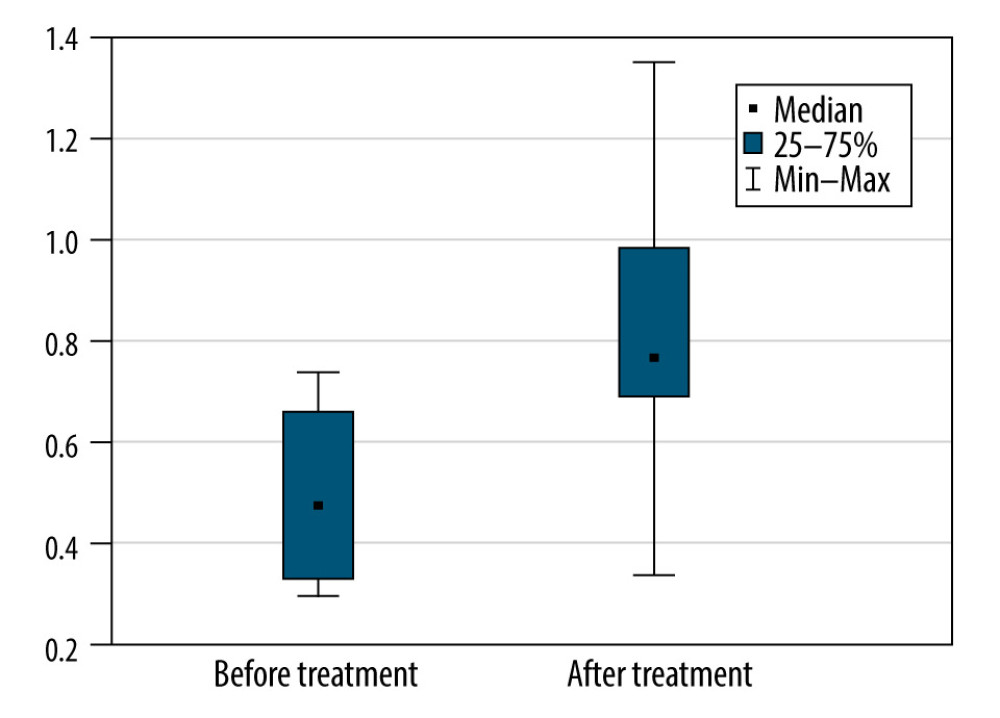 Figure 5. The monthly decrease in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and after treatment for the participants with a slow rate of progression of amyotrophic lateral sclerosis (P=0028).
Figure 5. The monthly decrease in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and after treatment for the participants with a slow rate of progression of amyotrophic lateral sclerosis (P=0028). References
1. Dash RP, Babu RJ, Srinivas NR, Two-decade-long journey from riluzole to edaravone: Revisiting the clinical pharmacokinetics of the only two amyotrophic lateral sclerosis therapeutics: Clin Pharmacokinet, 2018; 57(11); 1385-98
2. Mazzini L, Fagioli F, Boccaletti R, Stem cell therapy in amyotrophic lateral sclerosis: A methodological approach in humans: Amyotroph Lateral Scler Other Motor Neuron Disorder, 2003; 4(3); 158-61
3. Staff NP, Madigan NN, Morris J, Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS: Neurology, 2016; 87(21); 2230-34
4. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis: Arch Neurol, 2010; 67(10); 1187-94
5. Thomsen GM, Gowing G, Svendsen S, The past, present and future of stem cell clinical trials for ALS: Exp Neurol, 2014; 262(Pt B); 127-37
6. Syková E, Rychmach P, Drahorádová I, Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: Results of phase I/IIa clinical trial: Cell Transplant, 2017; 26(4); 647-58
7. Petrou P, Gothelf Y, Argov Z, Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: Results of phase 1/2 and 2a clinical trials: JAMA Neurol, 2016; 73(3); 337-44
8. Nabavi SM, Arab L, Jarooghi N, Safety, feasibility of intravenous and intrathecal injection of autologous bone marrow derived mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: An open label phase I clinical trial: Cell J, 2019; 20(4); 592-98
9. Barczewska M, Grudniak M, Maksymowicz S, Safety of intrathecal injection of Wharton’s jelly-derived mesenchymal stem cells in amyotrophic lateral sclerosis therapy: Neural Regen Res, 2019; 14(2); 313-18
10. Siwek T, Maksymowicz W, Barczewska M, Mesenchymal stem cell (MSC) transplantation in patients with amyotrophic lateral sclerosis (ALS): Is there a “responder population”?: J Neurol Neurosci, 2018; 9(3); 260
11. Grisendi G, Annerén C, Cafarelli L, GMP-manufactured density gradient media for optimized mesenchymal stromal/stem cell isolation and expansion: Cytotherapy, 2010; 12(4); 466-77
12. Dominici M, Le Blanc K, Mueller I, Minimum criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement: Cytotherapy, 2006; 8(4); 315-17
13. Cedarbaum JM, Stambler N, Malta E, The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III): J Neurol Sci, 1999; 169(1–2); 13-21
14. Kollewe K, Mauss U, Krampfl K, ALSFRS-R score and its ratio: A useful predictor for ALS progression: J Neurol Sci, 2008; 275(12); 69-73
15. Maksymowicz S, Kukołowicz P, Siwek T, Validation of the revised Amyotrophic Lateral Sclerosis Functional Rating Scale in Poland and its reliability in conditions of the medical experiment: Neurol Sci, 2020 [Online ahead of print]
16. Gugliandolo A, Bramanti P, Mazzon E, Mesenchymal stem cells: A potential therapeutical approach for amyotrophic lateral sclerosis?: Stem Cells Int, 2019; 2019 3675627
17. Czarzasta J, Habich A, Siwek T, Stem cells for ALS: An overview of possible therapeutic approaches: Int J Dev Neurosci, 2017; 57; 46-55
18. Barczewska M, Maksymowicz S, Zdolińska-Malinowska I, Umbilical cord mesenchymal stem cells in amyotrophic lateral sclerosis: An original study: Stem Cell Rev Rep, 2020; 16; 922-32
19. Kim HY, Kim H, Oh KW: Stem Cells, 2014; 32(10); 2724-31
20. Oh KW, Moon C, Kim HY, Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis: Stem Cells Transl Med, 2015; 4(6); 590-97
21. Oh KW, Noh MY, Kwon MS, Repeated intrathecal mesenchymal stem cells for amyotrophic lateral sclerosis: Ann Neurol, 2018; 84(3); 361-73
Figures
 Figure 1. The monthly decrease in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and after treatment for all participants (P=0.57).
Figure 1. The monthly decrease in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and after treatment for all participants (P=0.57). Figure 2. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period, based on data from the pretreatment observation period. The rate of progression decreased. (Arrows indicate injections.)
Figure 2. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period, based on data from the pretreatment observation period. The rate of progression decreased. (Arrows indicate injections.) Figure 3. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period, based on data from the pretreatment observation period. The rate of progression was unchanged. (Arrows indicate injections.)
Figure 3. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period, based on data from the pretreatment observation period. The rate of progression was unchanged. (Arrows indicate injections.) Figure 4. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period based on data from the pretreatment observation period. The rate of progression increased. (Arrows indicate injections.)
Figure 4. The monthly change in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and during treatment, and the linear trend function for the treatment period based on data from the pretreatment observation period. The rate of progression increased. (Arrows indicate injections.) Figure 5. The monthly decrease in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and after treatment for the participants with a slow rate of progression of amyotrophic lateral sclerosis (P=0028).
Figure 5. The monthly decrease in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores before and after treatment for the participants with a slow rate of progression of amyotrophic lateral sclerosis (P=0028). In Press
05 Apr 2024 : Clinical Research
Comparative Analysis of Transoral Endoscopic Parathyroidectomy Vestibular Approach and Focused Open Surgery...Med Sci Monit In Press; DOI: 10.12659/MSM.944128
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952